You are here
FamiCord: Europe’s largest cord blood bank and largest manufacturer of cord tissue MSC therapy
FamiCord Group is a network of cord blood banks and marketing partners that offers family storage across Europe and owns 8 stem cell processing laboratories. The head of FamiCord Group is the bank PBKM in Poland. With the acquisition of Stemlab (dba Crioestaminal) in Portugal, finalized in Sept. 2018, FamiCord has the largest inventory of newborn stem cells in Europe. The combined inventory of cord blood and other biological materials at FamiCord banks exceeds 355,000 as of Sept. 2018.
FamiCord Network for Banking Cord Blood and Tissue | |||
FamiCord Member or Partner | Lab | Location | Countries Served |
Yes | Warsaw, Poland | Poland | |
Yes | Lugano, Switzerland | Switzerland, Italy | |
Yes | Bucharest, Romania | Romania | |
Yes | Sulzbach, Germany | Various countries | |
No | Belgrade, Serbia | Serbia, Albania (& Kosovo), Bosnia (& Herzegovina), Croatia, Macedonia (FYROM), Montenegro | |
Yes | Stockholm, Sweden | Sweden, Denmark | |
No | Riga, Latvia | Latvia | |
Yes | Cantanhede, Portugal | Portugal, Italy, Spain, Switzerland | |
Yes | Krakow, Poland | Poland | |
No | Zurich, Switzerland | Various countries | |
No | Milan, Italy | Italy | |
Izvorna Celica (part of Bio Save) | No | Ljubljana, Slovenia | Slovenia |
Yes | Budapest, Hungary | Hungary | |
Yes | Montvale, NJ, USA | Various countries | |
Yes | Barcelona, Spain | Spain | |
No | Lviv, Ukraine | Ukraine | |
Yes | Ankara, Turkey | Turkey | |
The key to the financial success of the FamiCord Group is that they prefer the American business model of promoting annual storage fees for privately stored newborn stem cells. This lowers the upfront costs to the clients and ensures continuous income to the bank. Most cord blood banks in Europe follow a business model of charging families upfront for both the processing plus the entire long term storage. The banks that have no storage fees are at a disadvantage when the market contracts, or if they seek to be acquired, because their inventory does not generate revenue.
Parent’s Guide to Cord Blood Foundation visited FamiCord headquarters at Polski Bank Komórek Macierzystych (PBKM) in September 2018. The central PBKM laboratory for family banking, as well as their research laboratory, are co-located in a large building near Warsaw’s Chopin airport. The FamiCord Group has released 45 cord blood units from their family banks for both standard transplants and regenerative medicine therapies.
What is less well known about FamiCord, but perhaps more important: PBKM is Europe’s leading provider of therapies with Mesenchymal Stromal Cells from the Wharton’s Jelly of umbilical cord tissue (WJ-MSC). By July 2018, PBKM had supplied WJ-MSC therapies to nearly 1,000 patients, for a variety of diagnoses that are displayed in a pie chart below.
Under EU regulations, WJ-MSC for regenerative medicine are considered to be an Advanced Therapy Medicinal Product or ATMP. This product can only be exported from Poland as part of a registered clinical trial. Within Poland, the Main Pharmaceutical Office gives Hospital Exemptions (ATMP-HE) to offer compassionate WJ-MSC therapy to patients with serious conditions. Each patient case must be approved by the bioethical committee before the cellular therapy can be prepared for that patient. Only hospitals within Poland are eligible for the exemption from the national authorities, but foreign patients can enter Poland to receive this therapy. Once the hospital has received approval to treat a patient, the hospital signs a contract with the patient’s family that specifies exactly what type of cells will be delivered, the route of administration, the timing of their delivery, and the cell dose.
Since PBKM was granted the ATMP-HE manufacturing license in 2014, more than 20 hospitals in Poland have treated patients with WJ-MSC from PBKM. These patients were monitored for adverse events at the time of treatment, and efforts have been made to follow them and obtain retrospective reports on the efficacy of the WJ-MSC therapy. Since the ATMP-HE protocol is different from a clinical trial, there is no control group and the patients cannot be required to follow uniform procedures.
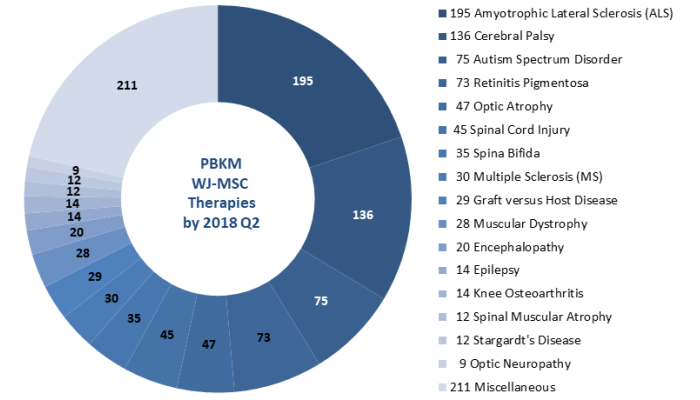

Amyotrophic Lateral Sclerosis (ALS) is the largest patient diagnosis group treated with WJ-MSC manufactured by PBKM. ALS is a progressive neurodegenerative disease which causes loss of muscle control and eventually patient death. PBKM has received a 3 million Euro grant from Poland’s national center for research and development (NCBiR) to develop a WJ-MSC treatment for ALS called “ALSTEM”, as well as a testing panel to identify patients that are the best candidates for the therapy. A publication reporting the research results is in press.
Spinal Muscular Atrophy is a different neurodegenerative disorder with fewer patients treated. A video is available on YouTube showing the dramatic improvement of patient Agata Roczniak (Polish only).
 Spina Bifida is also the topic of a paper submitted by physicians collaborating with PBKM. Spina Bifida is a birth defect in which the neural tube around the baby’s spinal cord does not close properly during in utero development. The standard of care for this defect is to operate shortly after birth to close the neural tube. The current procedure of the WJ-MSC therapy is to give the cells intravenously after the spina bifida surgery to support recovery. Even though pre-clinical studies have shown that, during intravenous administration MSC are quickly filtered by the lungs, nonetheless most of the spina bifida patients who received WJ-MSC have significant improvements in their digestion and bladder control. A video of Spina Bifida patient Amelka is available on YouTube (English sub-titles).
Spina Bifida is also the topic of a paper submitted by physicians collaborating with PBKM. Spina Bifida is a birth defect in which the neural tube around the baby’s spinal cord does not close properly during in utero development. The standard of care for this defect is to operate shortly after birth to close the neural tube. The current procedure of the WJ-MSC therapy is to give the cells intravenously after the spina bifida surgery to support recovery. Even though pre-clinical studies have shown that, during intravenous administration MSC are quickly filtered by the lungs, nonetheless most of the spina bifida patients who received WJ-MSC have significant improvements in their digestion and bladder control. A video of Spina Bifida patient Amelka is available on YouTube (English sub-titles).
Neurological conditions that are seen in young children and that have been treated with WJ-MSC from PBKM include Cerebral Palsy (136 patients) and Autism Spectrum Disorder (75 patients). PBKM presented a poster at the EBMT conference reporting that two thirds of the cerebral palsy patients experienced improved function after WJ-MSC therapy. In these treatments the children received either intravenous injections or intrathecal injections, and sometimes both, and further work is needed to evaluate patient response versus route of administration and dose.
 Orthopedic applications of WJ-MSC include knee cartilage repair. Boguslaw Sadlik, MD PhD, a surgeon at St. Luke’s hospital in Bielsko-Biala Poland, has published a procedure for repairing knee cartilage by dry arthroscopic implantation of a scaffold seeded with WJ-MSC. A video of the knee surgery is available on YouTube, as well as a testimonial from the patient (both in English).
Orthopedic applications of WJ-MSC include knee cartilage repair. Boguslaw Sadlik, MD PhD, a surgeon at St. Luke’s hospital in Bielsko-Biala Poland, has published a procedure for repairing knee cartilage by dry arthroscopic implantation of a scaffold seeded with WJ-MSC. A video of the knee surgery is available on YouTube, as well as a testimonial from the patient (both in English).
The WJ-MSC doses that are manufactured by PBKM start with umbilical cords that are donated by mothers once they have received health screening and given informed consent. When the umbilical cord arrives at the PBKM laboratory, a technician prepares it under a hood in a clean room. The donated umbilical cord is cut into tiny pieces and placed in culture flasks together with growth medium, a process called the “explants” method of isolating MSC from the Wharton’s Jelly. The WJ-MSC that grows out from the cords is harvested at the third passage and cryogenically frozen.
When a patient requires WJ-MSC therapy, a unit in the PBKM inventory is thawed and prepared as an injection holding the target cell dose. This injection is then hand carried to the hospital by a medical courier, inside a thermally insulated container with a temperature logger. Typically one patient receives WJ-MSC cells from only one donor. Sometimes if a larger dose is needed, the only way to have enough cells at third passage is to combine WJ-MSC from more than one donor. Although PBKM endeavors to track the donor family to check the health status of the baby and its mother, ultimately cord tissue stored in family banks can provide a more secure way to know the complete health history of the donor.
FamiCord Group is currently involved in 7 clinical trials at stage II and/or III, that will use WJ-MSC to treat a variety of conditions including epidermolysis bullosa, ischemic injuries of the cardiovascular system, muscular dystrophy, type 1 diabetes, and others. In total, around 700 patients will be enrolled in these trials.
PBKM provides additional laboratory services to the public health sector within Poland. They operate a public cord blood bank which currently holds over 3300 donations and has released one unrelated cord blood transplant. PBKM also prepares bone marrow and peripheral blood for stem cell transplants within Poland, so that the list of all PBKM stem cell therapies totals 1883 patients by the end of Sept. 2018.
FamiCord is clearly the leader to watch in terms of either family cord blood banking in Europe or the development of WJ-MSC therapies in the western hemisphere.


