You are here
News
2026
Mar 2026 From Bank to Bedside is a series of profiles that describe organizations which offer consumers the full range of cellular therapy services, from biobanking to clinical medicine. This month we profile CryoCord in Malaysia.
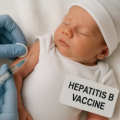
Mar 2026 The Hepatitis B vaccine (HBV) is given at birth to protect infants against a prevalent virus that causes lifelong illness and is the leading cause of liver cancer.
Feb 2026 Many parents are confused about the revised vaccine schedule published by the CDC. Parents should be reassured that, at least for 2026, their ability to vaccinate their children will not change. The American Academy of Pediatrics (AAP) is encouraging families to continue to follow the standard schedule for childhood vaccines. Insurance providers have guaranteed that they will cover the cost of vaccines through 2026.
Feb 2026 Profile of cord blood banking in Hungary, a country with a strong social network designed to encourage families to have children.
Jan 2026 When their daughter was diagnosed with Thalassemia, the parents banked cord blood from her younger brother. Two years later those sibling stem cells provided a life saving transplant. This story demonstrates both the power of cord blood banking, as well as the value of international networks to connect families, banks, and transplant centers.
Jan 2026 Within the United States, whole blood donors are not paid, versus donors of blood derivatives are paid. To avoid blood shortages, it may be time to reconsider "payments" to blood donors. Other countries employ a wide variety of incentives that are not cash payments.
2025
Dec 2025 Outside on a golf course, a kid can be a kid. The H.E.A.R.T.S. Club at Hillandale Golf Course provides support and respite to the families of children going through cell therapy at Duke University.
Dec 2025 The US government plans to reduce reimbursements for skin substitutes made of placenta. This has turned into a political topic, with millions of dollars spent on lobbying and many reversals of policy. We started covering this story in January 2025 and have major updates posted through December 2025.
Dec 2025 Zbigniew “Roger” Mrowiec, PhD, has retired from serving as Laboratory Director of the cell therapy facilities at Vitalant New Jersey (originally known as Community Blood Services). Under Roger’s leadership, the lab was responsible for many firsts. This memoir tells those stories so that the record will not be lost.
Nov 2025 Akshoy is the first child in India to have a successful cord blood transplant for thalassemia after conception of a savior sibling through PGD IVF. This was made possible by the Save The Sibling project that brings together expertise in reproductive medicine, stem cell science, and clinical transplantation.