You are here
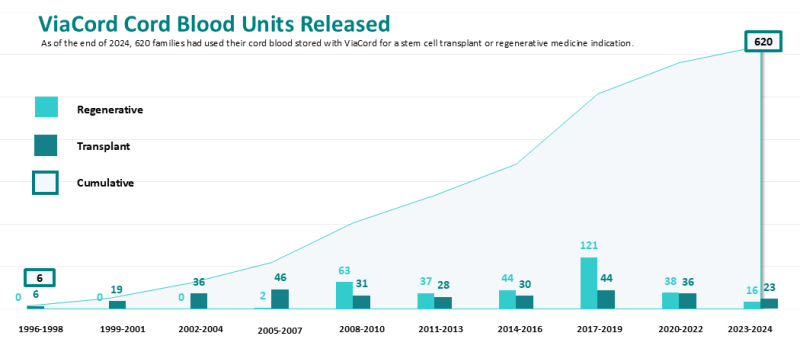
Over 620 cord blood units released by ViaCord®
Timeline of cord blood units released
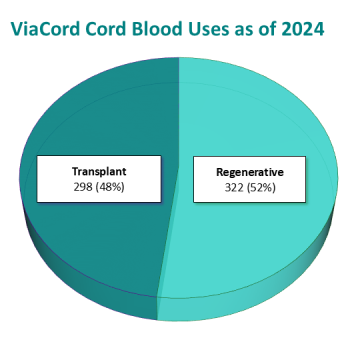
As of the end of 2024, 620 families had used their cord blood stored with ViaCord for a stem cell transplant (48%) or regenerative medicine indication (52%)1. The full list of treatments released is posted online. The graph below shows both ViaCord releases versus time as well as the cumulative releases to date.
Indications for releases
 Cord blood has been a reliable, effective, and life-saving source of stem cells in transplants for over 30 years2. Cord blood can be used in stem cell transplants for nearly 80 conditions to regenerate a healthy blood and immune system3. These conditions include hematological and immune system disorders, certain cancers, and metabolic diseases. Typically, a child with leukemia or a genetic disease who is in need of a transplant would require a cord blood unit from a sibling or an unrelated donor. However, there are some conditions, like the cancer neuroblastoma, where a child could use his or her own cord blood.
Cord blood has been a reliable, effective, and life-saving source of stem cells in transplants for over 30 years2. Cord blood can be used in stem cell transplants for nearly 80 conditions to regenerate a healthy blood and immune system3. These conditions include hematological and immune system disorders, certain cancers, and metabolic diseases. Typically, a child with leukemia or a genetic disease who is in need of a transplant would require a cord blood unit from a sibling or an unrelated donor. However, there are some conditions, like the cancer neuroblastoma, where a child could use his or her own cord blood.
Regenerative Medicine aims to harness the body's natural healing mechanisms by using living, healthy cells to repair, replace, or regenerate damaged cells, tissues, or organs to restore normal function. While regenerative medicine is an advancing and exciting field with great research interest, not all proposed indications have FDA approval or are considered standard of care. Many ViaCord families have had the chance to receive regenerative medicine therapies by participating in clinical trials using cord blood for certain conditions.
Conditions treated in ViaCord releases for stem cell transplants:
- 49% - Hemoglobinopathy (examples: Sickle Cell Disease, Thalassemia)
- 24% - Oncology (examples: acute leukemia)
- 18% - Bone Marrow Failure (examples: Fanconi Anemia, aplastic anemia)
- 7% - Immunodeficiency (example: chronic granulomatous disease)
- 2% - Metabolic Disorder (example: adrenoleukodystrophy)
Conditions treated in ViaCord releases for regenerative medicine:
- 97.5% - Brain Injury/Dysfunction (examples: cerebral palsy, autism, apraxia)
- 1.8% - Auto Immune condition (example: type 1 diabetes)
- 0.7% - Other (example: surgery for congenital heart defect)
Autologous versus Allogeneic use
Using one’s own stem cells (autologous) versus a donor source of stem cells (allogeneic) is determined by specific and multiple factors, including the patient’s condition and the availability of a compatible donor (match) adequate to be considered for clinical use. Ultimately, the treating physician will make the final decision.
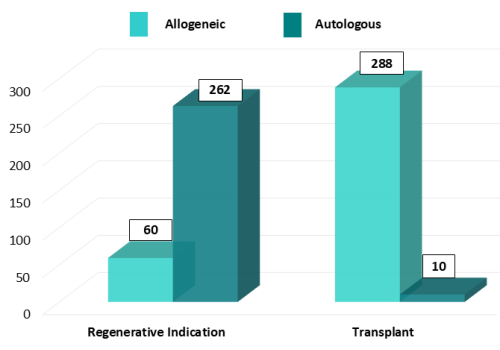
Of the 298 transplants released by ViaCord through the end of 2024, 97% were from a sibling donor, and only 3% were autologous therapies used by the child that banked the cord blood. Whereas, among the 322 regenerative medicine therapies, 81% were used by the child that banked the cord blood and 19% were allogeneic use by a sibling or other family member.
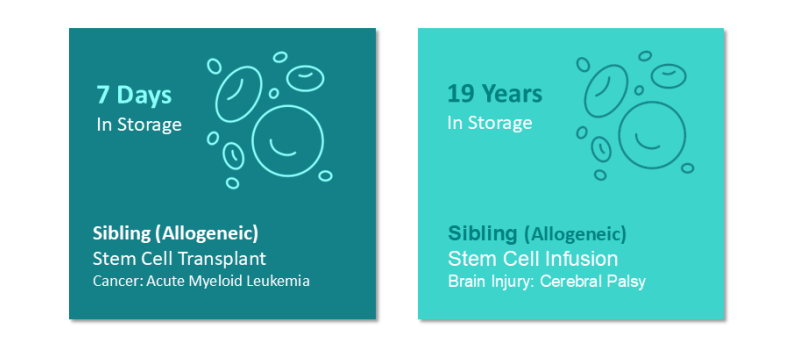
Demonstrating the Value of Cord Blood Banking
Cord blood continues to be a valuable resource for families – whether it is used days or decades after banking. Evidence to date indicates that properly cryopreserved and stored cord blood does not expire. In fact, researchers have demonstrated that cord blood stem cells retain their ability to function effectively after nearly three decades in cryogenic storage.4,5 ViaCord has successfully released cord blood samples ranging in storage age from 7 days to 19 years.
Watch ViaCord Videos from families who have used their cord blood and learn more about ViaCord's services, including the Sibling Connection program, at www.ViaCord.com.
References
- ViaCord Cord Blood Uses. Data On File as of 12/31/2024.
- Hao QL, Shah AJ, Thiemann FT, Smogorzewska EM, Crooks GM. A functional comparison of CD34 + CD38- cells in cord blood and bone marrow. Blood. 1995; 86(10):3745-3753.
- Parent's Guide to Cord Blood Foundation. Standard Therapies with blood-forming stem cells.
- Broxmeyer H, Luchsinger L, Weinberg R, Jimenez A, Frenet EM, van't Hof W, Capitano M, Hillyer C, Kaplan M, Cooper S, Ropa J, Abstract 16: Insights into Highly Engraftable Hematopoietic Cells from 27-Year Cryopreserved Umbilical Cord Blood. Stem Cells Translational Medicine. 2023; 12(s1):S18.
- Liedtke S, Többen S, Gressmann H, Meyer A, Verde PE, Gluckman E, Kogler G. Long-Term Stability of Cord Blood Units After 29 Years of Cryopreservation: Follow-Up Data From the José Carreras Cord Blood Bank. Stem Cells Translational Medicine. 2024; 13(1):30-42.
Disclaimer:
Banking cord blood does not guarantee that treatment will work and only a doctor can determine when it can be used.


