You are here
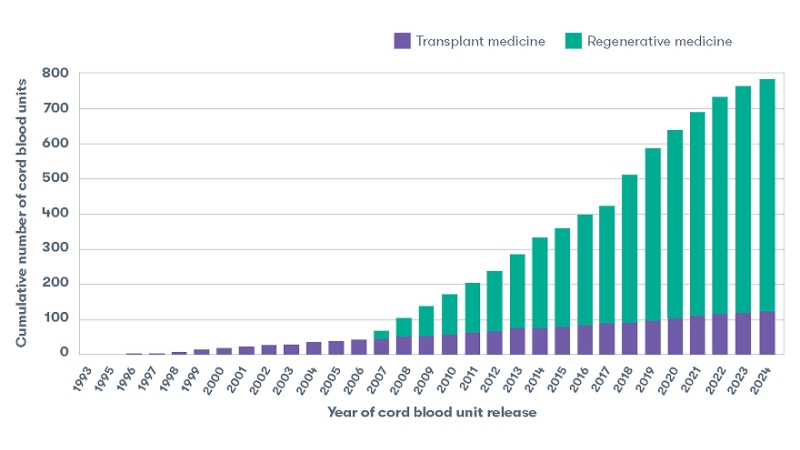
Over 750 cord blood units released by CBR
Cumulative cord blood units released1
Since CBR’s first cord blood unit release in 1993, we continue to provide families access to their newborn stem cells for both approved uses in transplant medicine and investigational uses in regenerative medicine. By the end of 2024, CBR’s release list had over 750 cord blood units – the most of any other family bank in the United States.1
Indications for releases1
Cord blood may be used as a graft source for a hematopoietic stem cell transplant, a procedure used to reconstitute healthy blood and immune systems. A stem cell transplant can treat over 80 conditions including hematological and immune system disorders, certain cancers, and metabolic diseases.2 CBR families' cord blood units have been released for intended use to treat over 20 different conditions. While blood disorders, including sickle cell anemia and beta thalassemia, are the most common category overall, the most common specific indication for transplant is acute lymphocytic/lymphoblastic leukemia (ALL).1
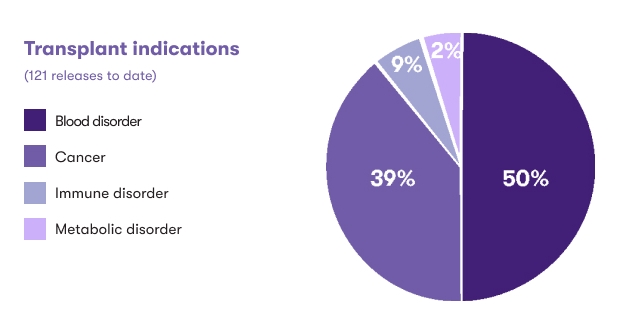
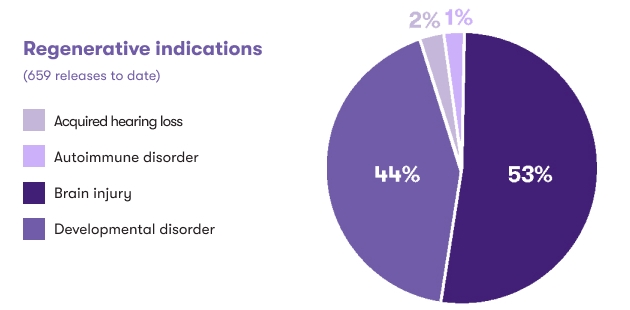
Cord blood is also being investigated in regenerative medicine, where the goal is to facilitate repair of damaged tissues and organs in the body. Through clinical trials and appropriately regulated protocols, 84% of CBR’s cord blood releases were intended for use in this growing area of medicine. Cerebral palsy and other related hypoxic/anoxic brain injuries are represented in the “Brain injury” category, with CBR having facilitated over 250 releases for these types of conditions.1
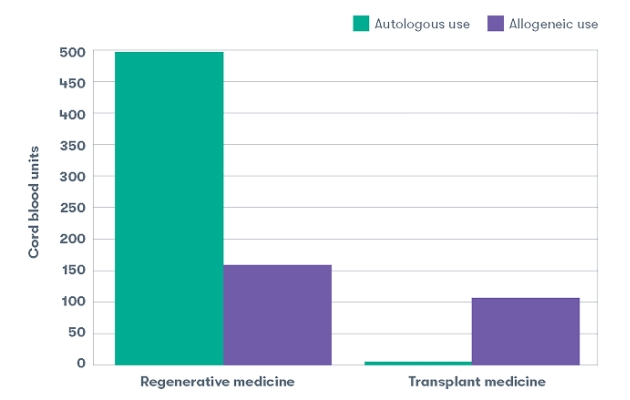
Autologous versus Allogeneic use1
Using one’s own stem cells (autologous) versus a donor source of stem cells (allogeneic) is always determined by the treating physician and is often dependent on the patient’s specific condition. Allogeneic use is common in the transplant medicine setting; 93% of our releases intended for use in a stem cell transplant were for allogeneic use. Autologous use is more common in regenerative medicine; 75% of our releases intended for use in investigational regenerative medicine applications were for autologous use.1
Age range of samples1
Samples stored for a duration of time ranging from 11 days to 17 years have been released by CBR.1 Over 200 releases have been facilitated for samples that had been in storage for 5 years or longer.1 Proper cryostorage helps ensure that cord blood samples remain viable for many decades.3

CBR’s Stem Cell Release Team
 CBR’s Stem Cell Release Team works with clinics and healthcare providers to ensure our clients have timely access to their newborn stem cell samples when a physician requests them for treatment. With over 30 years of release experience, CBR has earned a trusted name in the stem cell community and is proud to be recognized as the #1 OBGYN recommended newborn stem cell preservation company.1
CBR’s Stem Cell Release Team works with clinics and healthcare providers to ensure our clients have timely access to their newborn stem cell samples when a physician requests them for treatment. With over 30 years of release experience, CBR has earned a trusted name in the stem cell community and is proud to be recognized as the #1 OBGYN recommended newborn stem cell preservation company.1
While cumulative release data highlights CBR’s extensive experience in providing families with access to their baby’s newborn stem cells, individual stories are equally as important to celebrate. Check out our Family Stories page to hear about some of the incredible journeys of our clients, and how newborn stem cells have played a role.
About the author
Lauren Isley, MS, CGC is a Senior Clinical Science Specialist for Stem Cells at CooperSurgical. Lauren received her Masters of Science in Genetic Counseling from Wayne State University in 2011. She has worked in various women's health and fertility roles including preimplantation genetic testing, gamete donation, prenatal genetic testing, and newborn stem cells. Lauren currently serves as a Senior Clinical Science Specialist in the field of newborn stem cells and provides education and clinical support to internal and external audiences.
References
- CBR internal data on file
- Mayani H, Wagner JE, Broxmeyer HE. Cord blood research, banking, and transplantation: achievements, challenges, and perspectives. Bone Marrow Transplantation. 2020; 55:48–61.
- Broxmeyer HE, Luchsinger LL, Weinberg RS, et al. Insights into highly engraftable hematopoietic cells from 27-year cryopreserved umbilical cord blood. Cell Reports Medicine. 2023; 4(11):101259.
Disclaimers
The use of cord blood is determined by the treating physician and is influenced by many factors, including the patient's medical condition, the characteristics of the sample, and whether the cord blood should come from the patient or an appropriately matched donor. Cord blood has established uses in transplant medicine; however, its use in regenerative medicine is still being researched. There is no guarantee that potential medical applications being studied in the laboratory or clinical trials will become available.
Cord tissue use is still in early research stages, and there is no guarantee that treatments using cord tissue will be available in the future. Cord tissue is stored whole. Additional processing prior to use will be required to extract and prepare any of the multiple cell types from cryopreserved cord tissue. CBR Systems, Inc.’s activities for New York State residents are limited to collection of umbilical cord tissue and long-term storage of umbilical cord–derived stem cells. CBR Systems, Inc.’s possession of a New York State license for such collection and long-term storage does not indicate approval or endorsement of possible future uses or future suitability of these cells.


