You are here
Results from the Duke ACT Study of Cord Blood for Autism: Highlights for Parents
Introduction:
 Researchers at Duke University have been studying whether intravenous infusions of cord blood stem cells can help young children with autism spectrum disorder (ASD). The leaders of this research group are Dr. Joanne Kurtzberg and Dr. Geraldine Dawson.
Researchers at Duke University have been studying whether intravenous infusions of cord blood stem cells can help young children with autism spectrum disorder (ASD). The leaders of this research group are Dr. Joanne Kurtzberg and Dr. Geraldine Dawson.
An initial study (Duke ABC study) from this group published in 2017 treated 25 children ages 2-6 years with stem cells from their own cord blood. In that study, approximately 70% of the children had improvement in one or more core symptoms of autism. Importantly, children with non-verbal IQs (NVIQ) over 70 had more improvement from the cord blood therapy than children with lower IQs.
Based on those findings, the Duke group performed a second study (Duke ACT study) that was published in the Journal of Pediatrics in May 2020. This article highlights the key results.
Design of the Duke ACT Study:
The Duke ACT study was a randomized, double blinded, placebo controlled, prospective, cross-over study testing whether cord blood infusions would improve core symptoms of autism.
To be eligible for this study, children had to be between 2-7 years of age, have a confirmed diagnosis of autism spectrum disorder, be English speaking, and not have any known genetic anomalies. Applicants underwent a remotely administered test of cognitive function that was designed to only include children with non-verbal IQ above 70.
The study enrolled 180 children with autism and assigned them to three study arms.
- The Autologous arm held 56 children that received an infusion of stem cells from their own cord blood on their first visit to Duke. On their second visit 6 months later, these children received a placebo infusion.
- The Allogeneic arm held 63 children that received an infusion of stem cells from donated cord blood on their first visit to Duke. The donated cord blood was selected to match at least 4 out of 6 of the child’s HLA types. On their second visit 6 months later, these children received a placebo infusion.
- The Placebo arm held 61 children that received a placebo infusion on their first visit to Duke. The placebo was an inert infusion designed to look, feel, and even smell like a cord blood infusion. Then, on their second visit 6 months later, these children received cord blood stem cells. Half of the children on the Placebo arm received Autologous cord blood on the second visit and half received Allogeneic.
Notice that all of the children in this study received cord blood stem cells at some point, either at their initial visit or 6 months later. This is why it is called a “cross-over” study. But, it was also a “double blind” study, because neither the families nor their doctors knew which children were in which arm of the study. The study designers who did know this were careful to make sure that the children participating in the 3 arms were balanced in terms of their range of ages and IQs.
The children participating in the study traveled to Duke with their families and received their infusions in an outpatient setting. Each family traveled to Duke three times: for the initial therapy, for the second therapy 6 months later, and for a final follow up visit one year after they had entered the program. At each of these visits, the study children underwent a battery of skills testing and had imaging studies of their brain function.
Study results:
None of the children had a serious reaction to their infusions. About 5% of the children in the study experienced hives, cough, or wheezing after the infusion. Many of the participants felt anxious about the infusion, with between 40% and 50% of the families reporting psychiatric symptoms in their children in the first few weeks after their infusion.
Although the children participating in the study took a large battery of tests, as well as brain scans, the study was designed so that its “primary endpoint” was a single metric: whether or not cord blood therapy improved the participant’s mean score on the Vineland Adaptive Behavior Scales -3 (VABS-3) measure of socialization. This endpoint was not met for the study group as whole. Looking at all the participants on all 3 arms, the Duke ACT study did not show a benefit of cord blood over placebo on the VABS-3 Socialization Scale. However, there were some unexpected difficulties that weakened the study design, which are explained below in the next section.
The important positive outcome from the ACT study was that a subset of the children did show a significant benefit from cord blood therapy versus placebo, on multiple metrics. For the older children with higher IQ, improvements were seen in communication (VABS-3 Communication Scale), attention (eye tracking), and electroencephalogram (EEG) scans of brain connectivity. Also, the composite mean of scores in socialization and communication showed similar improvements for both of the cord blood arms (see the graph below).
Study difficulties:
The Duke ACT study encountered a few difficulties that hindered the ability of the study to demonstrate a benefit from cord blood therapy.
The biggest problem the ACT study encountered was that remotely administered IQ tests turned out to be less accurate than anticipated. The study was designed to ideally recruit 180 children with non-verbal IQ over 70, and could have been effective if only 143 children met the IQ threshold. Instead, only 101 of the study participants had non-verbal IQ over 70. This is why the study authors had to look at a subset of the participants in order to find clear benefits from cord blood therapy.
Another issue encountered in the ACT study was that score improvements among children on the “Placebo” arm were bigger than predicted. This made it harder to measure benefits of cord blood versus placebo. Remember that the only distinction between the study arms and the Placebo arm is that children in the Placebo group received cord blood stem cells 6 months later.
Conclusions and Next Steps:
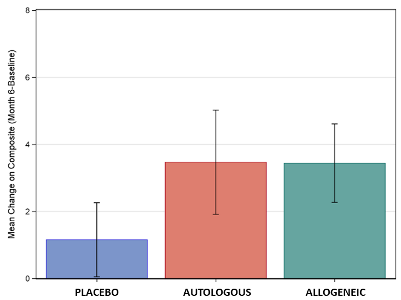 The graph displays the mean improvement in the composite score of socialization and communication for the three treatment groups, for the subset of children ages 4-7 with non-verbal IQ over 70. The vertical lines display the standard deviation around these means. Significant differences were seen between placebo and cord blood, but these scores did not show a difference between or Autologous or Allogeneic cord blood.
The graph displays the mean improvement in the composite score of socialization and communication for the three treatment groups, for the subset of children ages 4-7 with non-verbal IQ over 70. The vertical lines display the standard deviation around these means. Significant differences were seen between placebo and cord blood, but these scores did not show a difference between or Autologous or Allogeneic cord blood.
The results of this study will be applied to the design of future and ongoing clinical trials. Going forward, only children that can pass the IQ threshold during in-person testing will be enrolled in autism studies at Duke. For now, clinical trials must be restricted to those children with higher IQ where a benefit can clearly be proven. Other changes to future studies will be larger numbers of participants to compensate for the strong placebo improvements, and refined endpoints that look at the composite of socialization and communication scores on the VABS-3.
Duke recently opened a phase 2 randomized, placebo controlled, cross-over clinical trial testing umbilical cord tissue mesenchymal stromal cells (MSCs) to treat Autism Spectrum Disorder. The study was designed based on the lessons learned from Duke ACT. It will enroll 164 children, ages 4-8 years, with non-verbal IQ over 70.
The ACT study was funded by the Marcus Foundation. All of the costs were covered for the participants. Families were given a stipend towards travel for each study visit.


