You are here
Results from the Duke ACT Study of Cord Blood for Autism: The Inside Scoop from Dr. Kurtzberg
 Overview: The research groups in the Marcus Center for Cellular Cures and the Duke Center for Autism and Brain Development led by Drs. Joanne Kurtzberg and Geraldine Dawson, have been studying whether intravenous infusions of cord blood can improve the symptoms of autism in young children. They performed an initial study (Duke ABC study), published in Stem Cells Translational Medicine, for 25 children, ages 2-6 years, which demonstrated that autologous cord blood infusions were safe and that approximately 70% of children had improvement in one or more core symptom of autism. These improvements were correlated with changes in eye tracking, and brain scans of EEG and MRI in responding children. Importantly children with non-verbal IQs (NVIQ) >70 had more robust changes than children with lower IQs. Building on this study, a second clinical trial, The Duke ACT study, was designed to confirm these findings in a randomized study. The design and results of this study which was recently published in the Journal of Pediatrics are discussed below.
Overview: The research groups in the Marcus Center for Cellular Cures and the Duke Center for Autism and Brain Development led by Drs. Joanne Kurtzberg and Geraldine Dawson, have been studying whether intravenous infusions of cord blood can improve the symptoms of autism in young children. They performed an initial study (Duke ABC study), published in Stem Cells Translational Medicine, for 25 children, ages 2-6 years, which demonstrated that autologous cord blood infusions were safe and that approximately 70% of children had improvement in one or more core symptom of autism. These improvements were correlated with changes in eye tracking, and brain scans of EEG and MRI in responding children. Importantly children with non-verbal IQs (NVIQ) >70 had more robust changes than children with lower IQs. Building on this study, a second clinical trial, The Duke ACT study, was designed to confirm these findings in a randomized study. The design and results of this study which was recently published in the Journal of Pediatrics are discussed below.
Background: The Duke ACT study was a randomized, double blinded, placebo controlled, prospective, cross-over study testing whether cord blood infusions would improve core symptoms of autism in 180 affected children. Children were eligible if they were 2-7 years of age, with a confirmed diagnosis of ASD, without a known genetic cause of ASD, English speaking, and without other confounding illnesses. Children had to be tested and negative for Fragile-X and had to have a negative chromosomal microarray.
Based on results from the Duke ABC study which showed that children with a non-verbal IQ >70 had the most improvement after an infusion of autologous cord blood, children were screened remotely for cognitive function and attempts were made to only include children with nonverbal IQ>70. However, children that had traveled to Duke were not turned away from participation if their on-site IQ testing revealed a lower result than the remote IQ screening. The study was modelled to enroll 180 children with the expectation that a minimum of 143 would meet the criteria of NVIQ >70, but as a consequence of inaccurate remote IQ screening, only 101 children met this criteria, which impacts the results below.
Study Design: The study enrolled 180 subjects divided between treatment and placebo arms. Children received autologous cord blood if they had a qualifying unit that contained a minimum of 25 million cells per kg of the child’s weight, based on the pre-cryopreservation count. Children lacking an autologous cord blood unit received a >4/6 HLA matching unrelated donor cord blood unit obtained from the Carolinas Cord Blood Bank, a public FDA licensed cord blood bank at Duke. Both the autologous and donor cord blood units had to contain a minimum cell dose of 25 million cells per kg.
Children were stratified by age and non-verbal IQ. Ninety children each were assigned to the autologous and allogeneic arms. Each arm was individually randomized 2:1 for treatment versus placebo. Thus, 120 children received cord blood (60 autologous and 60 allogeneic) and 60 children received placebo for their first infusion. Children were analyzed for response at 6 months after their first infusion. Children crossed over at 6 months to a second infusion. Children receiving cord blood for their first infusion received placebo for their second infusion. Children receiving placebo for their first infusion received cord blood for their second infusion. The type of infusion given at baseline and at 6 months was blinded so that no one interacting with or testing the children knew which infusion was given at each time-point.
The primary endpoint of the study was to look for a change in the mean score on the Vineland Adaptive Behavior Scales -3 (VABS-3) Socialization Scale at 6 months post treatment. Key secondary endpoints included any change in the mean of the VABS-3 communication scale score, the Clinical Global Impression – Improvement scale, the Expressive one-word picture vocabulary test, and the PDD Behavior Inventory. Exploratory endpoints included changes in whole brain connectivity on MRI, EEG, and Eye tracking.
Treatment: All children were treated in the outpatient setting. Every infusion consisted of a pink liquid containing the cryopreservative DMSO, which causes a funny smell on the breath for several hours after the infusion. The cord blood unit was thawed on the day of infusion, washed and reconstituted in dextran/albumin in a volume of 1.25mL per kg for infusion. Children were pre-medicated with a single dose (0.5mg per kg each) of IV Benadryl and solumedrol at 30-60 minutes prior to the cord blood or placebo infusion. Cord blood or the placebo were infused over 15 minutes followed by a 1-hour infusion of standard IV fluids. The placebo consisted of TC199 + 1% DMSO.
Safety Results: There were no significant severe adverse events (SAEs) observed during the study. Mild infusion reactions consisting of hives, cough or wheezing, occurred in 4/61 children in the placebo group, 2/56 children in the autologous cord blood group, and 3/63 children in the allogeneic cord blood group. None were serious and all resolved with additional treatment with Benadryl, albuterol, and/or a second dose of solumedrol. Many parents reported mild and transient anxiety or other psychiatric symptoms in their children post infusion (27/61 placebo, 22/56 autologous, 30/63 allogeneic) but these did not differ between the groups.
Efficacy Results: Per the rules of clinical trials, the primary endpoint has to be analyzed regardless of whether the study design was successful or not. The analysis of the whole study population for Duke ACT did not show a benefit of cord blood over placebo on the VABS-3 Socialization Scale, which was the primary endpoint of the study. However, this analysis was flawed because only 101 of the 180 children enrolled on the study had a NVIQ >70, whereas the minimum number of subjects needed for this analysis was 143 children with NVIQ >70. In addition, the strong improvements seen in children on the placebo arm, where children received a placebo infusion first followed by a cord blood infusion 6 months later, also compromised the study analysis and the subsequent interpretation of the results.
Improvements were seen in communication (VABS-3 Communication Scale), attention (eye tracking), and increased alpha and beta EEG power. In most of the measures, there were no advantages of allogeneic or autologous cord blood identified. But on the Clinical Global Impression-Improvement scale, only children receiving allogeneic cord blood showed improvement, compared to placebo (this graph is in the publication). It is very important to note that children receiving allogeneic cord blood were given a higher total dose of cells compared to the children receiving autologous cord blood. Thus, it was not possible to know whether this ‘advantage’ of allogeneic cord blood was due to increased dose or the donor cell source.
Table of Scores: The table below displays the mean improvements (and their standard deviation) in scores for several behavioral measures, broken down to compare the children by NVIQ group and by type of cell therapy received. The composite social/communication improvement displays the mean of the social score improvement and the communication score improvement for that group. In the final three rows of the table, score changes are shown only for children in the age range 4-7 years and with NVIQ >70. The older children (ages 4-7 years) with higher IQ (NVIQ >70) had the best response to the cord blood therapy (these scores are highlighted in pink in the table).
Mean (Standard Deviation) Change in VABS III Standard Scores, Baseline to Month 6 | ||||
NVIQ | Treatment | Socialization | Communication | Mean of |
<70 | Allogeneic (N=33) | 3 (6.4) | 3.76 (11.61) | 3.38 (8.33) |
Autologous (N=20) | 1.3 (9.77) | 1.15 (7.03) | 1.23 (7.53) | |
Placebo (N=22) | 1.82 (8.08) | 7.09 (11.5) | 4.45 (8.48) | |
≥70 | Allogeneic (N=30) | 3 (7.75) | 2.87 (6.48) | 2.93 (6.04) |
Autologous (N=36) | 4.31(10.76) | 3.03 (9.08) | 3.67 (8.61) | |
Placebo (N=35) | 2.09 (8.73) | 0.11 (7.29) | 1.1 (6.02) | |
|
| |||
≥70, Ages 4-7
| Allogeneic (N=26) | 3.31 (7.99) | 3.58 (6.25) | 3.44 (5.97) |
Autologous (N=33) | 4.06 (11.14) | 2.88 (9.44) | 3.47 (8.94) | |
Placebo (N=32) | 1.56 (8.77) | 0.75 (7.17) | 1.16 (6.25) | |
There were three lessons learned from this study:
(1) The placebo effect was higher than predicted in the parent reported outcomes (VABS-3).
(2) Remote measurement of IQ is not a reliable screen, especially for children under 4 years of age.
(3) It was challenging to measure response in lower functioning children with currently available tools.
Accordingly, these observations will be applied to the design of future and ongoing trials. Specifically, going forward, we will restrict eligibility to children 4-7 years with full scale IQ >70. We will have the study team measure IQ on-site prior to enrollment. This will mean, though, that some children will come for testing and then be turned away because they don’t meet final study eligibility. We will refine the primary endpoint of the clinical trial to encompass the mean of socialization and communication scales on the VABS-3. We also acknowledge a definite and absolute need for a concomitant placebo-control, cross-over arm. We also have to increase the number of patients in the study (sample size) to account for the large placebo effect.
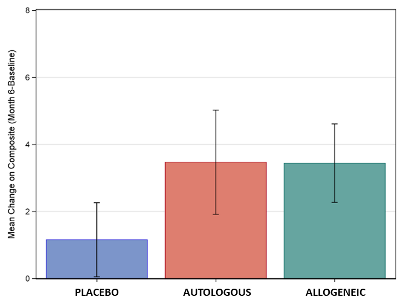 Graph of Scores: The figure displays the mean improvement in the composite score of socialization and communication for the three treatment groups, for those children ages 4-7 with non-verbal IQ>70. Significant differences were seen between placebo and cord blood, but there were no differences between allogeneic or autologous cord blood.
Graph of Scores: The figure displays the mean improvement in the composite score of socialization and communication for the three treatment groups, for those children ages 4-7 with non-verbal IQ>70. Significant differences were seen between placebo and cord blood, but there were no differences between allogeneic or autologous cord blood.
This study was funded by the Marcus Foundation. All costs associated with testing, obtaining cord blood, and preparing infusions, were covered by the study. Families were given a stipend towards travel for each study visit. Duke recently opened a phase 2 randomized, placebo controlled, cross-over clinical trial testing human cord tissue mesenchymal stromal cells (MSCs) to treat Autism Spectrum Disorder. The study was designed based on the lessons learned from Duke ACT. It will enroll 164 children, ages 4-8 years, with non-verbal IQs>70. Future studies are planned to test this population of children with cord blood infusions as well.


