You are here
The Maternal Side of the Placenta Has Valuable Cells Too
What is the Placenta Decidua?
The placenta is an amazing organ that mediates exchanges between the mother and her baby during pregnancy1. It is small but mighty. When a pregnancy is full term, the typical placenta weighs about one pound (a half kg) and passes 20 ounces (0.6 liter) of blood per minute.
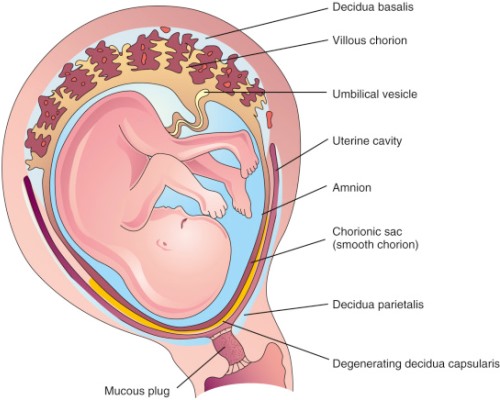 Most of the placenta forms from the fertilized egg and is a genetic match to the baby. However, a small part of the placenta is maternal in origin and medical professionals refer to the placenta as a “maternal-fetal organ”2. So that the fertilized egg may attach to the wall of the mother’s uterus, the lining of the uterus grows fingers which interlock with fingers extending from the fetal placenta, and together they anchor the placenta (see the diagram). The portion of the uterus where the lining thickens and forms fingers extending into the placenta is called the Placenta Decidua and it is composed of tissues that match the mother3.
Most of the placenta forms from the fertilized egg and is a genetic match to the baby. However, a small part of the placenta is maternal in origin and medical professionals refer to the placenta as a “maternal-fetal organ”2. So that the fertilized egg may attach to the wall of the mother’s uterus, the lining of the uterus grows fingers which interlock with fingers extending from the fetal placenta, and together they anchor the placenta (see the diagram). The portion of the uterus where the lining thickens and forms fingers extending into the placenta is called the Placenta Decidua and it is composed of tissues that match the mother3.
The maternal and fetal sides of the placenta communicate throughout pregnancy. Researchers have found that the placenta decidua secretes the hormone prolactin, which travels across the amnion-chorion to bathe the fetus in high levels of prolactin throughout pregnancy. The prolactin plays an important role in maintaining maternal-fetal immune tolerance, so that the mother’s immune system does not attack the baby during the pregnancy3.
When we talk about companies that are collecting placenta donations for the manufacture of medical products, so far, those companies have all been working with the fetal side of the placenta4-6. The mother’s consent to placenta donation covers the entire placenta, but it is easier to work with the fetal side. The fetal side is bigger, it is easier to harvest a piece of tissue that is clearly of fetal origin, and the fetal side has not been exposed to everything in the mother’s medical history. However, a tiny number of maternal and fetal cells do cross the placenta in either direction. It is known that you can find a few cells in a mother’s body that match the genetics of one of the children she carried decades later7. Some scientists have cautioned that when fetal placenta cells are culture expanded, it is possible that a few maternal cells may grow out too8.
The power of Decidua Stromal Cells (DSC)
A recently published paper has concluded that Decidua Stromal Cells are the best cellular therapy for Graft versus Host Disease (GvHD), a complication of stem cell transplants that is often fatal9. These Decidua Stromal Cells (DSC) are a special name for Mesenchymal Stromal cells (MSC) harvested from the placenta decidua.
The authors of the study promoting DSC are Olle Ringdén, MD PhD, and Behnam Sadeghi, MD PhD, and they are affiliated with the Karolinska Institutet in Huddinge, Sweden9. The historical context is that researchers at the Karolinska Institutet were the very first to treat GvHD with MSC in 2004, saving the life of a nine-year-old child with severe acute GvHD10. In the 20 years since then, many clinical studies have used MSC, mostly derived from bone marrow, to treat GvHD. But MSC have only achieved approval in three countries as a GvHD therapy that can be given outside of clinical trials11. This is another example of a cellular therapy where some patients have reported remarkable recoveries, and yet the overall statistics of patient benefit from late phase clinical trials has not been strong. A very comprehensive review of MSC for GvHD published in 2023 pointed out that the previous meta-analyses have not sorted the MSC by tissue source, and that more work is needed to find biomarkers that predict which GvHD patients will have a strong response12.
Here are the numbers that explain why Ringdén & Sadeghi say that DSC are a “new frontier” in GvHD therapy: The complication of GvHD occurs in about 37% of patients after a stem cell transplant13. In clinical trials of MSC therapy for patients with steroid-resistant GvHD, typically two thirds of the patients respond to MSC, and two thirds of those responders survive six months later9,12. By comparison, when Ringdén & Sadeghi tested DSC on 21 patients with severe acute GvHD, 100% of the patients responded, and 81% of the patients had survived one year later. This was a small patient group, but the results are notable.
How are DSC different from other MSC?
While most tissues of the human body contain cells that meet the definition of MSC, it is important to emphasize that they do not all behave the same clinically. In other words, the MSC source matters. The DSC from the placenta decidua are similar to other MSC sources in having an excellent safety profile, with only rare and mild infusion effects reported14-16. The DSC from the placenta decidua are different in that they are about half the size of bone marrow MSC, they are much more likely to trigger coagulation when given intravenously, and they have a stronger immunosuppressive effect than other sources of MSC, but their immunosuppression is also more dependent on cell-to-cell contact9,17-19.
It is exciting to see that cells from the maternal side of the placenta are also getting recognition. In addition to GvHD, placenta decidua DSC have also been used in clinical trials for hemorrhagic cystitis and acute respiratory distress syndrome (ARDS)20,21. Hopefully Ringdén & Sadeghi can arrange for larger clinical trials of DSC which can lead to their approval for use in larger populations of patients.
References:
- Ben-Senior L. 10 Amazing Facts About the Placenta. Parent's Guide to Cord Blood Foundation Newsletter Published 2020-10
- Sapanara N. What your placenta can and can't do. Parent's Guide to Cord Blood Foundation Newsletter Published 2020-02
- Flores-Espinosa P, Méndez I, Irles C, Olmos-Ortiz A, Helguera-Repetto C, Mancilla-Herrera I, Ortuño-Sahagún D, Goffin V, Zaga-Clavellina V. Immunomodulatory role of decidual prolactin on the human fetal membranes and placenta. Frontiers Immunology. 2023; 14:1-11.
- Schweizer R. Amniotic Membrane of the Placenta - Part 1. Parent's Guide to Cord Blood Foundation Newsletter Published 2016-09
- Verter F. It’s the morning before your C-section, and someone wants you to donate your placenta. Parent's Guide to Cord Blood Foundation Newsletter Published 2021-01
- Tibbot T, Verter F. What do they do with all those placenta donations? Parent's Guide to Cord Blood Foundation Newsletter Published 2023-12
- Dawe GS, Tan XW, Xiao ZC. Cell Migration from Baby to Mother. Cell Adhesion & Migration. 2007; 1(1):19-27.
- Silini AR, Parolini O. Are we ready for placenta cells to enter the clinic? Parent's Guide to Cord Blood Foundation Newsletter Published 2018-06
- Ringdén O & Sadeghi B. Placenta-Derived Decidua Stromal Cells: A New Frontier in the Therapy of Acute Graft-Versus-Host Disease. Stem Cells. 2024; 42(4):291–300.
- Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M, Ringdén O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. The Lancet. 2004; 363(9419):1439-1441.
- Robb KP, Galipeau J, Shi Y, Schuster M, Martin I, Viswanathan S. Failure to launch commercially-approved mesenchymal stromal cell therapies: what's the path forward? Cytotherapy. 2024; 26(5):413-417.
- Kadri N, Amu S, Iacobaeus E, Boberg E, Le Blanc K. Current perspectives on mesenchymal stromal cell therapy for graft versus host disease. Nature, Cellular & Molecular Immunology. 2023; 20:613–625.
- Arai S, Arora M, Wang T, Spellman SR, He W, Couriel DR, .... Pavleti SZ. Increasing Incidence of Chronic Graft-versus-Host Disease in Allogeneic Transplantation: A Report from the Center for International Blood and Marrow Transplant Research. Biol. Blood Marrow Transpl. 2015; 21(2):266-274.
- Thompson M, Mei SHJ, Wolfe D, Champagne J, Fergusson D, Stewart DJ, ... McIntyre L. Cell therapy with intravascular administration of mesenchymal stromal cells continues to appear safe: An updated systematic review and meta-analysis. eClinicalMedicine. 2020; 19:100249.
- Sadeghi B, Moretti G, Arnberg F, Samén E, Kohein B, Catar R, ... Ringdén O. Preclinical Toxicity Evaluation of Clinical Grade Placenta-Derived Decidua Stromal Cells. Frontiers Immunology. 2019; 10:2685.
- Baygan A, Aronsson-Kurttila W, Moretti G, Tibert B, Dahllöf G, Klingspor L, ... Ringdén O. Safety and Side Effects of Using Placenta-Derived Decidual Stromal Cells for Graft-versus-Host Disease and Hemorrhagic Cystitis. Frontiers Immunology. 2017; 8:795.
- Moll G, Ignatowicz L, Catar R, Luecht C, Sadeghi B, Hamad O, ... Ringdén O. Different Procoagulant Activity of Therapeutic Mesenchymal Stromal Cells Derived from Bone Marrow and Placental Decidua. Stem Cells and Development. 2015; 24(19)
- Erkers T, Nava S, Yosef J, Ringdén O, Kaipe H. Decidual Stromal Cells Promote Regulatory T Cells and Suppress Alloreactivity in a Cell Contact-Dependent Manner. Stem Cells and Development. 2013; 22(19)
- Ringdén O, Moll G, Gustafsson B, Sadeghi B. Mesenchymal Stromal Cells for Enhancing Hematopoietic Engraftment and Treatment of Graft-Versus-Host Disease, Hemorrhages and Acute Respiratory Distress Syndrome. Frontiers Immunology. 2022; 13:839844
- Aronsson-Kurttila W, Baygan A, Moretti G, Remberger M, Khoein B, Moll G, Sadeghi B, Ringdén O. Placenta-Derived Decidua Stromal Cells for Hemorrhagic Cystitis after Stem Cell Transplantation. Acta Haematologica. 2018; 139(2):106–114.
- Sadeghi B, Roshandel E, Pirsalehi A, Kazemi S, Sankanian G, Majidi M, .... Hajifathali A. Conquering the cytokine storm in COVID-19-induced ARDS using placenta-derived decidua stromal cells. J Cellular Molecular Med. 2021; 25(22):10554-64.


