You are here
Amniotic Membrane of the Placenta - Part 1
 DOWNLOAD Amniotic Membrane Products spreadsheet
DOWNLOAD Amniotic Membrane Products spreadsheet
Amnion tissue, an integral part of the human placenta, has been used in therapy for many years. As a very thin and elastic membrane lining the inside of the placental sac, this unique creation has a long history of topical wound healing, and it’s therapeutic benefits continue to be identified and it’s uses continue to grow. As we tell our story about amnion, our first installment will focus on today’s uses. Future articles will discuss why we believe the amniotic membrane of the placenta is a ‘paradigm shifter’ for regenerative medicine and new cellular therapies, followed by our report on what we see as tomorrow’s amnion-based products.
Amnion begins as a sheath around the umbilical cord, evolving during pregnancy to become a thin inner lining of the placental sac. Amnion is often used whole as a wound covering. Amnion is also a source of Mesenchymal Stem Cells (MSC’s).
It wasn’t too long ago that there was a common belief that MSC’s were substantially equivalent whether found in bone marrow, adipose tissue, the umbilical cord, or placental tissue. However we’re seeing new clinical data citing differences in MSC’s based on their origin and other factors. Hence we now distinguish between cells derived from amniotic membrane of the placenta (AMP), versus the thicker and more robust amnion that sheaths the umbilical cord. We call the Mesenchymal-like Stem Cells found in the AMP "Amnion-derived Stem Cells" (ADSCs).
The following is a basic description of key AMP properties that begin to explain their many years of use, and continued growth in the therapeutic application as tissue or cellular derivative:
- Comprises collagen, fibronectin and hyaluronic acid that enhances wound healing.
- Comprises a combination of growth factors, cytokines and anti-inflammatory proteins that typically aid cell-to-cell communication in immune responses.
- Evidence the tissue exhibits anti-inflammatory, antifibroblastic, and antimicrobial properties.
- Considered non-immunogenic: not observed to cause substantial immune response, thus considered for allogeneic uses.
- The stromal side adjacent to the chorion of the placental sac has a population of fetal-based ADSC’s in situ.
- The opposite side adjacent to the amniotic fluid and the fetus comprises a layer of Epithelial Stem Cells (AEC’s) in situ.
AMP has been used dermally for wound care for many years and it appears that dehydration and/or cryopreservation of the tissue does not compromise its healing capability. Our work at AmnioChor has also demonstrated that we can cryopreserve living tissue and retain cellular viability post thaw.
Figure 1 below is a diagram of FDA regulations on amnion products. The abbreviations 361 and 351 refer to sections of the FDA code regulating human cell, tissue and cellular and tissue-based products (HCT/Ps). Very briefly, a “361 product” is one that is minimally manipulated and intended for autologous use (donor and recipient are the same), and homologous use (similar structure to it's origin). Products that can squeeze under the 361 exemption can go from bench to bedside more easily and with much less expense. Any cell therapy product in which cells have been cultured, combined with other articles, are intended for non-homologous use, etc., is considered to be a “351 product” in which the cell therapy is regulated the same as a drug.
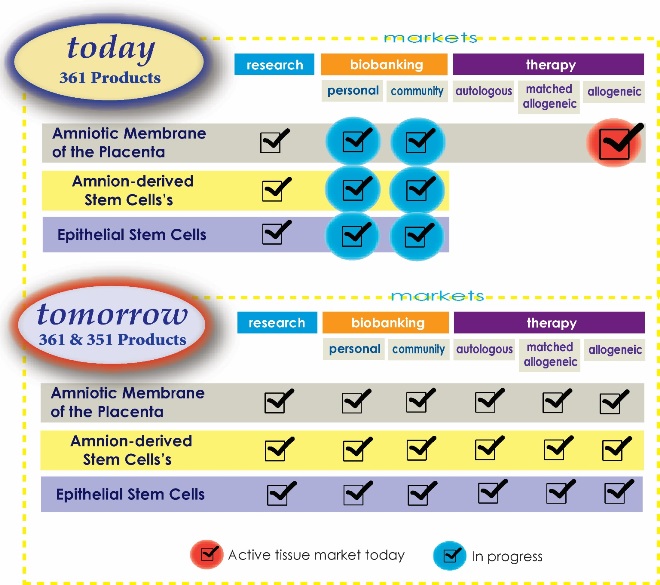
Right now, there’s really only one viable AMP therapy product market, as highlighted by the red check mark in Figure 1. It’s based on tissue utilization under the 361 exemption, and comprised of four market segments:
- Orthopedic, Sports and Spine
- General, Gynecology and Urology
- Wound Care
- Ophthalmology
At AmnioChor, we have been working to create tissue bank of both private and public AMP storage as highlighted by the blue check marks in Figure 1. We feel there’s great need and value in cryopreserving this tissue for future therapies that are either autologous, matched allogeneic, or unmatched allogeneic treatments. We will discuss this in much greater detail in Part 2 of this article series.
The financial value of the current AMP tissue market for the four segments noted is estimated to be about $450 million in 2016, and expected to grow at a rate of over 15% to 2020. Wound Care comprises over 60% of this market today, growing at a slightly lower rate than the aggregate. Ortho, Sports and Spine, at a little over 20% of the amnion market today, is expected to grow the fastest over the next four years.
Perhaps we should interject some important FDA clarity by stating that these products are all considered by their manufacturer to be eligible for the 361 exemption. However we know that on occasion, the term FDA clarity is an oxymoron, and in this case, all the AMP products shown are for allogeneic use, not autologous, and thus do not meet the traditional definition of the 361 exemption. So far it appears the FDA is willing to allow the HCT/P allogeneic use of AMP for dermal products. The regulatory status of amnion products is a very large and evolving topic that deserves a more comprehensive discussion. Also, certain FDA meetings scheduled for September 2016 may bear heavily on future policy. For example, at the FDA hearing on Sept 12 many participants requested that the concept of homologous use be extended to cover not just structural origin but functional origin. Therefore we will defer a discussion of amnion regulations to a later installment of this article series.
We need to make one more FDA related comment. There is a notable group of what we’ve called ‘flowable’ products that typically comprise solutions of micronized AMP tissue and a variety of other wellness ingredients in liquid form. Most are topically applied for dermal application, however several are injectable. We’ve tried to steer away from describing clinics that offer amnion injectable products, including amniotic fluid based products. These activities are clearly not legal under current FDA regulations.
 Looking back again at Figure 1, private biobanking of amnion tissue or stem cells does not exist today. However we recommend that personal storage of AMP will be a valuable choice when it becomes available. Parents will be able to bank multiple samples that will allow multiple therapy opportunities. There may be some specialized therapies available in the future that require less tissue, such as periodontal, ocular, and others yet to be identified, where better outcomes may be achieved from autologous and/or matched allogeneic tissue.
Looking back again at Figure 1, private biobanking of amnion tissue or stem cells does not exist today. However we recommend that personal storage of AMP will be a valuable choice when it becomes available. Parents will be able to bank multiple samples that will allow multiple therapy opportunities. There may be some specialized therapies available in the future that require less tissue, such as periodontal, ocular, and others yet to be identified, where better outcomes may be achieved from autologous and/or matched allogeneic tissue.
Next month, look for our second installment titled: “Amniotic Membrane of the Placenta, Part 2 - Can it be a Paradigm Shifter?” We will begin to discuss the role that AMP plays in a holistic model of hybrid perinatal cell banking and therapy.


