You are here
Cartistem® Cord Blood-Derived Therapy for Knee Arthritis
Medipost is a South Korean company that has developed a pipeline of cell therapy products in which the active cells are allogeneic Mesenchymal Stromal Cells (MSC) derived from cord blood1. This approach is a bit unusual. During the first dozen years (2005-2017) of clinical trials with MSC from perinatal sources, most of the trials sourced their cells from birth tissues, with only 14% of trials using MSC sourced from blood2. But Medipost adopted their technology platform early, and has diligently pursued clinical trials of their products since 20093.
 The Medipost strategy paid off in January 2012 when their Cartistem® product was approved by the South Korea Ministry of Food and Drug Safety (MFDS) for the treatment of knee cartilage defects caused by osteoarthritis degeneration or by repetitive trauma4. This approval made Cartistem® the first regenerative medicine product manufactured from cord blood to achieve approval anywhere in the world.
The Medipost strategy paid off in January 2012 when their Cartistem® product was approved by the South Korea Ministry of Food and Drug Safety (MFDS) for the treatment of knee cartilage defects caused by osteoarthritis degeneration or by repetitive trauma4. This approval made Cartistem® the first regenerative medicine product manufactured from cord blood to achieve approval anywhere in the world.
Cartistem® is surgically administered as injections into the knee at a target dose of 2.5 x 106 cells/500㎕/cm2. Specifically, Cartistem® is approved for grade IV cartilage defects in which the cartilage is torn and exposes the subchondral bone5. Worldwide, 365 million people had knee arthritis in 2019, and the global numbers keep increasing as populations age6,7. Orthopedic doctors employ a variety of strategies to treat knee cartilage defects, with osteochondral allografts reserved for the bigger and deeper lesions5,8. Studies disagree on the exact prevalence of grade IV chondral defects, but approximately 5-10 % of people over age 40 have high grade chondral lesions8.
 The publicly traded company Medipost has experienced great success providing Cartistem® to patients with severe knee osteoarthritis. In 2022, a publication about the 5-year follow-up of patients treated with Cartistem® received the annual award for best original research from the journal of the American Orthopaedic Society for Sports Medicine (AOSSM)9. By June of 2024, the number of patients treated with Cartistem® had surpassed 30,00010. The Korea Biomedical review reports that “Cartistem sales have grown at an annual growth rate of 36 percent through 2023, becoming the first Korean stem cell therapy to exceed 20 billion won ($14.4 million) in annual sales”10.
The publicly traded company Medipost has experienced great success providing Cartistem® to patients with severe knee osteoarthritis. In 2022, a publication about the 5-year follow-up of patients treated with Cartistem® received the annual award for best original research from the journal of the American Orthopaedic Society for Sports Medicine (AOSSM)9. By June of 2024, the number of patients treated with Cartistem® had surpassed 30,00010. The Korea Biomedical review reports that “Cartistem sales have grown at an annual growth rate of 36 percent through 2023, becoming the first Korean stem cell therapy to exceed 20 billion won ($14.4 million) in annual sales”10.
Medipost is a full spectrum cell therapy company with activities that range from biobanking to clinical research and development. Medipost operates one of the largest hybrid cord blood banks in South Korea, under the brand name CellTree. As of October 2024, their net inventory was 319 thousand cord blood units, and they had released 563 transplants (468 unrelated)11.
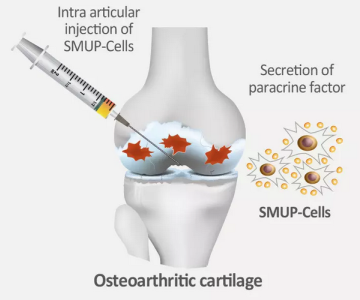 Medipost is seeking to grow their therapeutic market by getting approval of Cartistem® for additional orthopedic indications. In December of 2024, Korean regulators rejected a request to approve Cartistem® for the treatment of ankle joint talar cartilage and osteochondral defects12. The verdict was that the phase 2 trial data showing clinical efficacy of Cartistem® for this indication were not strong enough. Medipost intends to try again with a phase 3 trial12. Meanwhile, Medipost has also developed a second-generation version of Cartistem®, currently known as SMUP-1A-01, which combines small (SM) cell selection with their proprietary culture method to produce an ultra-potent (UP) cell therapy13. According to Medipost, this second-generation version of cord blood-derived MSC can be manufactured with higher efficiency and lower cost. The SMUP-1A-01 product is in a phase 2 clinical trial in South Korea which employs intra-articular injections to treat and prevent damage from osteoarthritis13.
Medipost is seeking to grow their therapeutic market by getting approval of Cartistem® for additional orthopedic indications. In December of 2024, Korean regulators rejected a request to approve Cartistem® for the treatment of ankle joint talar cartilage and osteochondral defects12. The verdict was that the phase 2 trial data showing clinical efficacy of Cartistem® for this indication were not strong enough. Medipost intends to try again with a phase 3 trial12. Meanwhile, Medipost has also developed a second-generation version of Cartistem®, currently known as SMUP-1A-01, which combines small (SM) cell selection with their proprietary culture method to produce an ultra-potent (UP) cell therapy13. According to Medipost, this second-generation version of cord blood-derived MSC can be manufactured with higher efficiency and lower cost. The SMUP-1A-01 product is in a phase 2 clinical trial in South Korea which employs intra-articular injections to treat and prevent damage from osteoarthritis13.
Medipost is also seeking to grow their therapeutic market by leveraging their success in South Korea to obtain market approvals in additional countries. Medipost is currently running clinical trials to obtain approvals for Cartistem® as a knee therapy in Japan and in North America.
 In Japan, Medipost launched a phase 2 clinical trial in December 2019 that would have evaluated the efficacy of Cartistem® in combination with High Tibial Osteotomy (HTO). However, the Coronavirus pandemic hindered enrollment in that trial. In February of 2024, Medipost received permission from Japan’s Pharmaceuticals and Medical Devices Agency (PMDA) to withdraw the phase 2 trial and focus instead on a phase 3 trial of Cartistem® alone14. That phase 3 trial had achieved 90% enrollment by June 202410. Cartistem® is manufactured in Japan by the company Evastem, a joint venture between Medipost and the Japanese pharmaceutical company VICX therapeutics15.
In Japan, Medipost launched a phase 2 clinical trial in December 2019 that would have evaluated the efficacy of Cartistem® in combination with High Tibial Osteotomy (HTO). However, the Coronavirus pandemic hindered enrollment in that trial. In February of 2024, Medipost received permission from Japan’s Pharmaceuticals and Medical Devices Agency (PMDA) to withdraw the phase 2 trial and focus instead on a phase 3 trial of Cartistem® alone14. That phase 3 trial had achieved 90% enrollment by June 202410. Cartistem® is manufactured in Japan by the company Evastem, a joint venture between Medipost and the Japanese pharmaceutical company VICX therapeutics15.
In Canada, Medipost invested CAD 90 million in May 2022 to partner with OmniaBio Inc., a subsidiary of Canada’s Centre for Commercialization of Regenerative Medicine (CCRM)16. Together, Medipost and OmniaBio have built a new contract development and manufacturing organization (CDMO) in Hamilton, Ontario, Canada17. As reported by Business Korea, “OmniaBio now boasts a total production space of 11,200 m2, making it the largest specialized CDMO company for cell and gene therapies in Canada”17. Meanwhile, Medipost has completed a phase 1/2 clinical trial of Cartistem® in the United States10. They are skipping phase 2 and are preparing the Investigational New Drug (IND) application for a phase 3 clinical trial, with product manufacturing conducted by OmniaBio10,17. Medipost’s end goal is to achieve US FDA market approval for Cartistem®. With that end in mind, Medipost has recently hired an executive team to manage their North America operations18.
It is impossible to write an article about the success of Cartistem® without reflecting on the relative speed of cell therapy product approvals in the East versus the West. Cartistem® is one of a class of cell therapies that are manufactured by isolating and expanding specific cells derived from cord blood3. While Cartistem® was approved in South Korea in January 2012, it took 11 years, until April 2023, before the second approval of an expanded cord blood product. The second approval came when the US FDA approved Omisirge (formerly known as Omidubicel, and before that as NiCord) from Gamida Cell19. Whereas it took Cartistem® three years of clinical trials to gain approval in South Korea, Omisirge had been in multi-national trials for 16 years (since 2007) before it was finally approved3,19. Admittedly, Cartistem® is a regenerative medicine therapy for the prevalent condition arthritis, whereas Omisirge is designed to speed the engraftment of cord blood transplants, and consequently the trials of Omisirge involved sicker patients and had slower accrual. The approval of Omisirge came too late to prevent the bankruptcy of Gamida Cell in 202420. One cannot help wonder if patients today would have routine access to expanded cord blood transplants if Omisirge, and other similar products, had gone to market first in an Asian country. Thanks to the rapid uptake of Cartistem® in South Korea, the company is well positioned to get product approvals in other countries, so that in the near future with severe knee arthritis may have access to a biologic therapy derived from cord blood.
References
- Medipost. Products. First-Generation of Stem Cell Products.
- Verter F, Silva Couto P, Bersenev A. A Dozen Years of Clinical Trials Performing Advanced Cell Therapy with Perinatal Cells. Future Science 2018; 4(10):FSO351.
- Verter F, Bersenev A, Silva Couto P. Clinical Trials of Expanded Cord Blood. Parent's Guide to Cord Blood Foundation Newsletter Published 2018-05
- South Korea Ministry of Food & Drug Safety. Biological Products. CARTISTEM® Last updated 2016-03-24
- International Cartilage Repair Society (ICRS). Cartilage Repair Techniques. Accessed 2024-12-31
- World Health Organization (WHO). Osteoarthritis. Published 2023-07-14
- GBD 2021 Osteoarthritis Collaborators. Global, regional, and national burden of osteoarthritis, 1990–2020 and projections to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet Rhematology 2023; 5(9):e508-e522.
- Watts E, McCulloch PC. Articular Cartilage Defects of Knee. Ortho Bullets. Last updated 2024-12-06
- Medipost. MEDIPOST’s 5-year follow-up of phase 3 clinical trial patients receives 2021 OJSM award. Stem Cell Therapeutic. Press Release. Published 2022-07-18
- Kim C-H. Number of patients getting Cartistem tops 30,000: Medipost. Korea Biomedical Review. Published 2024-06-24
- Medipost. Business. CELLTREE®.
- Sung JJ. Medipost’s ‘Cartistem’ ankle treatment additional indication rejected by Ministry of Food and Drug Safety, plans to re-challenge for Phase 3 clinical trial. THE BIO. Published 2024-12-26
- Medipost. Products.Second-Generation of Stem Cell Products.
- Kim C-H. Medipost withdraws Japanese p2 trial for stem cell therapy. Korea Biomedical Review. Published 2024-02-05
- Lee H-S. Japan OKs Medipost's P2 trial of stem cell-serviced osteoarthritis treatment. Korea Biomedical Review. Published 2019-12-09
- OmniaBio. OmniaBio Inc. announces private investor. Press Release. Published 2022-05-31
- Choi M-H. Medipost's OmniaBio Launches Largest CGT CDMO Facility in Canada. Business Korea. Published 2024-10-21
- Medipost. MEDIPOST, Inc. Expands U.S. Presence with Key Executive Appointments, advances toward Phase III clinical trial of CARTISTEM® in North America. Press Release. Published 2024-12-03
- Verter F. 1st FDA Approval Omisirge Expanded Cord Blood. Parent's Guide to Cord Blood Foundation Newsletter Published 2023-05
- Cooley LLP. Gamida Cell Completes International Restructuring. Press Release. Published 2024-06-21


