You are here
Parent’s Guide to Whole Genome Sequencing
“Genomics” is the study of all the DNA in the human body, the full set of information that makes you who you are. Your DNA is located on 23 pairs of chromosomes. There is conflicting information on the internet about how many genes there are in the human genome. The reason for this confusion is that scientists are still working to identify genes. In the late 1990’s, there was a big race between two research consortiums to map the human genome1,2. Going into this race, scientists believed that humans had about 100,000 genes, one to code each protein in the human body. As a result of mapping the genome, scientists discovered that our genes only code pieces of the proteins. There are still competing research groups that maintain different databases of genes, but today the consensus is that we have about 22,000 genes3.
Genetic testing has become a routine part of childbirth in many developed nations. For example, in the United States, each state has a recommended uniform screening panel (RUSP) of genetic diseases for which infants are automatically tested at government expense, although there are big differences in the list of diseases screened from state to state4. Many family cord blood banks encourage parents to purchase additional genetic testing that goes beyond the basic RUSP lists. The focus is always on giving parents “actionable” information that identifies childhood conditions which can be prevented, treated, or managed more efficiently with early intervention5.
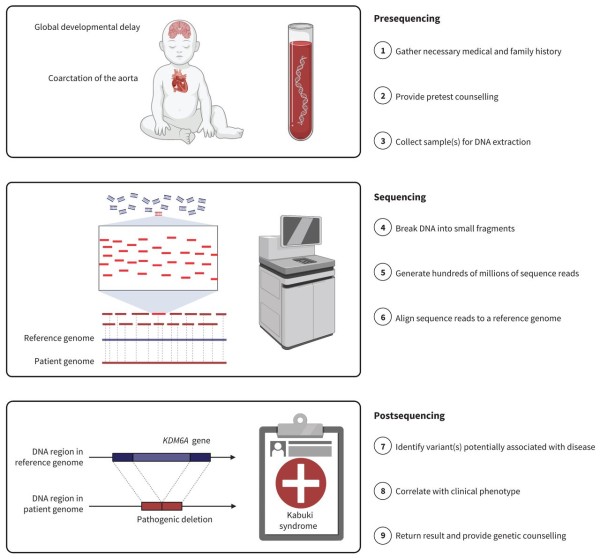
What is the difference between Whole Genome Screening (WGS) versus standard genetic testing? There are two main differences. The first difference is that WGS analyzes the entire genome instead of targeting specific genes. With WGS, it is easier to identify disorders that are caused by multiple genes acting in combination. There are currently over 7,000 genetic disorders identified, and the list is growing all the time6-8. The second difference is that WGS always uses the latest technology, which is called Next Generation Sequencing (NGS), whereas genetic tests for limited lists of conditions may rely on older methods. Studies have been run in which a large group of apparently healthy infants were tested by both WGS and by traditional genetic screening; the result was that WGS was able to find a significant number of pediatric-onset disorders that were missed by traditional screening tests9-11. The bottom line is that WGS is the most thorough and the most accurate form of genetic screening.
Here is an example of how today’s WGS technology can impact parents. The Parent’s Guide to Cord Blood was founded in memory of Dr. Verter’s first child. When she became pregnant with her second child in 1997, Verter asked her doctor to run screening tests for every known inherited disease. The doctor burst out laughing and said: “You don’t have enough blood for that!”. It was necessary to compromise. Verter and her husband met with a genetic counselor, who worked up a short list of diseases of highest concern based on their family histories, and ran tests for those. But today, with access to WGS, it would be possible for a new mother to fulfill that wish to test the parents or the baby for every known genetic disease.
For this article, we interviewed Dr. Madhuri Hegde, the chief scientific officer of Revvity, previously affiliated with PerkinElmer. The Revvity U.S. laboratory is one of the first commercial laboratories to launch WGS for healthy newborns. Revvity has also introduced a research workflow pipeline that enables other laboratories and newborn screening programs to outsource targeted gene sequencing to the Revvity laboratory12. While there are other companies in the business of marketing whole genome sequencing to parents, many of them are outsourcing their genetic testing to the Revvity laboratory.
Dr. Hegde says that over her 30 years of working in genetics, she has found it to be a deeply personal topic. Some people want to know everything about their genetics, whereas some people only want to get the information they absolutely need to know. These preferences are spelled out when clients fill out their consent form, and clients receive genetic counseling both before and after the testing.
When parents sign up for WGS of their children, there are limits to what they will be told. Genetic testing laboratories endeavor to follow the practice guidelines set by the American College of Medical Genetics (ACMG)13. The following information is available to parents:
- Genetic variances that are known to cause diseases in childhood
- Genetic variances that are known to create risk for childhood illness
- Pharmokinetic insights into drug sensitivities and allergies
The information listed above is sometimes called the WGS “actionable” findings, because having this knowledge enables the parents to take actions to safeguard their child’s health. The full genome information from the WGS is stored securely in case it needs to be re-analyzed. Once the child turns 18, they have autonomy to make their own decisions about how much genetic information they want to know. They can request the additional knowledge which is available to adult clients:
- Genetic variances that are known to cause diseases in adulthood
- Carrier status with regards to diseases that can be inherited
The child may not want to know all about their health risks at age 18. But later, when they contemplate being parents themselves, they may come back and say that they are ready to know everything. The ethical guideline for reporting findings from WGS is to inform the client about any genetic variations which are known to be “pathogenic”, in other words those variations that are known to be associated with a medical condition13-17. The clients will not receive information about “variations of unknown significance”.
Sometimes people are stunned to learn that they are a carrier of an inherited disease. This situation is surprisingly common. Most “rare disorders” (impacting less than 60 per 200,000 people) are genetic in nature, and collectively about 1 in 20 people are carriers of one of the rare genetic diseases18-20. Dr. Hegde says “Studies have indicated that individuals carry at least one to two pathogenic variants”.
The use of WGS is becoming increasingly prevalent in medicine because the cost of sequencing the entire genome has dropped drastically over the past quarter century. The NIH-funded effort to sequence the first human genome cost $2.7 billion in today’s dollars, whereas in 2024, the cost of WGS technology is approximately $600 per genome21. In fact, the technology costs have dropped so far that the price of the overall service is starting to reach a plateau that is driven mainly by the cost of skilled labor such as genetic counselors. By 2021, close to 30 million people had undergone WGS22.
One area where WGS is still working to improve is the diversity of the reference database. A genetic variant can only be recognized by comparison to a genetic standard. But there is so much genetic variation from one human being to the next that there is no such thing as a person with “standard” DNA that can be used as a baseline for comparison. WGS laboratories rely on a reference genome that is actually a combination of many people. For example, Revvity relies on the Genome in a Bottle (GIAB) reference from the National Institute of Standards and Technology (NIST)23. There are research consortia that are working to incorporate a wider variety of human genetics into the reference standards. The NIH is funding a program called All of Us that seeks to capture the genetic diversity of people in the U.S.24. Even more important is the international consortium called Human Heredity and Health in Africa (H3Africa), which seeks to collect genetic information from people in Africa22,25. Because Africa was the cradle of the human race, there is more genetic diversity within Africans than among all of the races that traveled out of Africa22. Gradually, as the reference genome becomes more diverse, we will have a better understanding of which variants have significance.
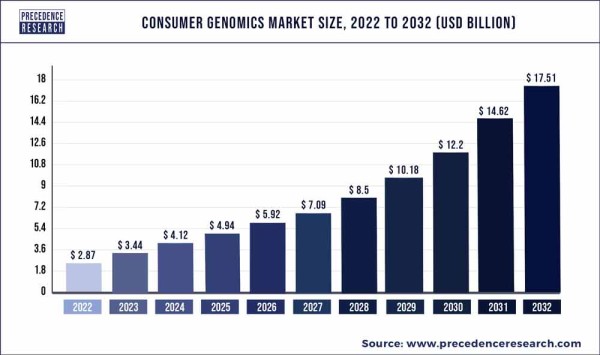
Consumers have been exploring their own genetics for a while now, through limited tests from services such as AncestryDNA or 23andMe. But the current price of WGS technology means that it is now possible to pay just a bit more and get more accurate testing of your entire genome, plus interpretation by a genetic counselor, from companies such as Revvity, Fore Genomics, Fulgent Genetics, and others. The current generation of direct-to-consumer WGS providers also offer security: your genomic data is never sold to third parties, it is not deposited to your medical records without your consent, and it is not stored on the internet where it can be hacked. Most importantly, direct-to-consumer WGS incorporates pre- and post-test counseling through certified genetic counselors. Experts are predicting that WGS will take over the consumer market for genetic testing.
Experts also predict that it is only a matter of time before WGS is incorporated into routine public health programs. The United Kingdom has embarked on a pilot study to perform WGS for 100,000 newborns26. When WGS is run for large numbers of patients, it can cost less than running traditional genetic screening for a limited list of conditions27,28. The traditional screening generates false positives and requires a lot of retesting. Also, traditional screening panels are cumbersome to update. These problems disappear when WGS is routinely performed: the testing is more accurate on the first pass, and additional tests can be run at the software level by reanalyzing the data. Government support of routine WGS would alleviate healthcare disparities that arise because different insurance policies provide different coverage for WGS expenses. When forecasting the future use of WGS in medicine, the big remaining questions are how will it be offered and who will pay for it?
References:
- Shreeve J. The Genome War: How Craig Venter Tried to Capture the Code of Life and Save the World. 2005 Published by Ballantine Books
- National Institutes of Health (NIH). The Human Genome Project. Last updated 2024-05-14
- Salzberg SL. Open questions: How many genes do we have? BMC Biology. 2018; 16:94.
- Brown H, & Verter F. Newborn Screening Complements Cord Blood Banking. Parent's Guide to Cord Blood Foundation Newsletter Published 2020-09
- Verter F. Actionable Genetic Tests. Parent's Guide to Cord Blood Foundation Newsletter Published 2020-04
- Bick D, Bick SL, Dimmock DP, Fowler TA, Caulfield MJ, Scott RH. An online compendium of treatable genetic disorders. Am J Med Genet C Semin Med Genet. 2021; 187(1):48–54.
- Online Mendelian Inheritance in Man. OMIM Morbid Map Scorecard. Last updated 2024-07-05
- Clinical Genome Resource. Classification Summary for 7410 Curated Variants Across 110 Genes. Curated Variants.
- Balciuniene J, Liu R, Bean L, Guo F, Nallamilli BRR, Guruju N, ... Hegde M. At-Risk Genomic Findings for Pediatric-Onset Disorders From Genome Sequencing vs Medically Actionable Gene Panel in Proactive Screening of Newborns and Children. JAMA Network Open. 2023; 6(7):e2326445.
- Guo F, Liu R, Pan Y, Collins C, Bean L, Ma Z, ... Hegde M. Evidence from 2100 index cases supports genome sequencing as a first-tier genetic test. 2023; Genetics in Medicine. 26(1):100995.
- Guo F, Liu R, Pan Y, Colasanto M, Collins C, Hegde M. Beyond Single Diagnosis: Exploring Multidiagnostic Realities in Pediatric Patients through Genome Sequencing. Human Mutation. 2024; 2024:9115364.
- Revvity. Revvity Introduces New Workflow to Accelerate Newborn Sequencing Research. Announcement. Published 2024-03-15
- American College of Medical Genetics and Genomics. Medical Genetics Practice Resources. Web Portal. Last updated 2024
- MedLine Plus. What are secondary findings from genetic testing? MedLine Plus. Last updated 2021-07-29
- ACMG Secondary Findings Working Group. Recommendations for reporting of secondary findings in clinical exome and genome sequencing, 2021 update: a policy statement of the American College of Medical Genetics and Genomics (ACMG). Genetics in Medicine. 2021; 23:1391-1398.
- 3 Billion Inc. What are the ACMG Standards and Guidelines and how do they work? 3billion blog. Published 2021-11-19
- Wolf SM. The Continuing Evolution of Ethical Standards for Genomic Sequencing in Clinical Care: Restoring Patient Choice. J Law Med Ethics. 2017; 45(3):333–340.
- Crain E. What is a rare disease? Johnson & Johnson health & wellness blog. Published 2023-02-08
- Wikipedia. Genetic Disorder.
- Jackson M, Marks L, May GHW, Wilson JB. The genetic basis of disease. Essays Biochem. 2018; 62(5): 643–723.
- 3 Billion Inc. Whole genome sequencing price and trends 2024. 3billion blog. Published 2023-12-18
- Crespi S. Looking back at 20 years of human genome sequencing. Science Podcast. Published 2021-02-04
- National Institute of Standards and Technology (NIST). Genome in a Bottle. NIST projects. Last updated 2024-04-16
- National Institutes of health. All of Us Research Program. Join All of Us. Copyright 2024.
- H3Africa – Human Heredity & Health in Africa. FAQ. Last updated 2023.
- Genomics England Limited. Newborn Genomes Programme. The Generation Study. Website copyright 2024.
- Powell CM. What Genomic Sequencing Can Offer Universal Newborn Screening Programs. Hastings Center Report. 2018; 48(Suppl 2):S18–S19.
- Costain G, Cohn RD, Scherer SW, Marshall CR. Genome sequencing as a diagnostic test. Canadian Medical Assoc J. 2021; 193(42):E1626-E1629.