You are here
News
2017
Mar 2017 LifeCell has launched a new approach that redefines family cord blood banking as "Community Stem Cell Banking".
Mar 2017 Cerebral Palsy Alliance proposes a five year study of to compare trials of cord blood, mesenchymal stem cells, and neural progenitor cells for CP patients.
Feb 2017 Congress taking place 19-21 May 2017 in Teaneck NJ carries 16 CME credits on evidence-based research and products derived from Perinatal sources.
Feb 2017 Despite the hurdles, great therapeutic value can be gained from the MSCs in the Wharton’s Jelly of Cord Tissue, which are being employed in an ever-increasing portfolio of indications.
Feb 2017 Available exclusively from Cordlife, CellOptima™ is a proprietary technology which can isolate and expand two types of stem cells: MSC and EpSC, from the lining of the newborn's umbilical cord.
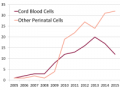
Jan 2017 There are more advanced cell therapy clinical trials with other sources of perinatal cells than there are with cord blood, either cumulatively or by year.
Jan 2017 When he was 12 days old Jack became the youngest person ever to undergo stem cell therapy in Canada and the first person in the country to be treated for HIE with stem cells.
2016
Dec 2016 Literature review finds umbilical cord clamping at 30-60 secs after term birth ensures safe outcome for baby and adequate cord blood collection.
Dec 2016 Fraction of cord blood collections suitable for transplant goes down by a factor of 7 when cord clamping delay exceeds 1 minute.
Nov 2016 Slides from Parent's Guide to Cord Blood Foundation at Phacilitate Cord Blood and Tissue Webinar 16 Nov. 2016. All presentations will be archived.