You are here
News
2020
Dec 2020 CellSave Arabia celebrates 15 years as the first and largest stem cell laboratory in the Gulf region. While CellSave Arabia primarily offers cord blood banking, they also seek new technologies and services for their customers. CellSave Arabia has partnered with the Abu Dhabi Stem Cell Centre (ADSCC), the healthcare centre that facilitated UAE's first stem cell transplant in summer 2020.
Nov 2020 LifeCell’s Community Cord Blood Bank has saved the life of an Indian child with aplastic anaemia. Two matching cord blood units were provided for transplant at no additional cost - saving lakhs of rupees for the family.
Nov 2020 If parents want to collect cord blood from a birth at one of the UK’s NHS public hospitals, it is mandatory to hire a phlebotomist. Also, in the UK NHS system, parents do not get to choose who will deliver their baby. The experience is a bit different from what families expect elsewhere, but cord blood banks in the UK have found creative ways to connect with parents to deliver education about newborn stem cells.
Nov 2020 Colt’s story shows that it is possible for older children to benefit from stem cell therapy. Colt had been diagnosed with both cerebral palsy and autism. His parents took him to a clinic offering therapy with cord tissue MSC when he was already 11 years old. After the therapy, Colt had notable improvements in his speech, balance, and sensory issues.
Oct 2020 Not only is the placenta an amazing organ during pregnancy, moreover its benefits extend beyond pregnancy and can offer hope that will last a lifetime.
Oct 2020 Cells4Life offers parents a choice of placenta banking services: amniotic membrane or chorionic tissue. Together with umbilical cord tissue, this gives parents access to different types of birth tissues and the unique cell populations that each contains.
Oct 2020 At present, 41% of recruiting cord blood trials are using a specialized cell product derived from cord blood, not just unmanipulated cord blood cells.
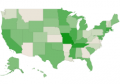
Sep 2020 Within the United States, mandatory newborn screening varies dramatically from state to state. Parents that want to screen their baby for all actionable genetic conditions can purchase supplemental newborn screening.
Sep 2020 Three days after a healthy birth, Jack Tilling nearly died. It took his parents and physicians two and a half years of searching to finally learn that Jack's diagnosis is a urea cycle disorder. Jack's diagnosis could have been detected with newborn screening.
Aug 2020 The AABB 2020 Virtual Annual Meeting will be held Oct. 3-5. Session topics include cellular and genetic therapies, sickle cell disease, pediatric care, hematopoietic stem cells, cord blood, platelet transfusion, genotyping, inventory management, biological product collection and utilization – and much, much more.