You are here
What happens to cord blood cells when the freezing process is interrupted?
The birth of a child is a one-time opportunity to harvest from the umbilical cord blood those stem cells that can rebuild and replenish a patient's blood and immune systems, and provide regenerative therapy. Unlike stem cells that come from bone marrow or peripheral blood, it is not possible to return to the source if the stem cells are lost or damaged. Thus proper freezing (cryopreservation) and storage for future transplantation are of the utmost importance for cord blood cells.
Freezing normally takes place in an instrument called a controlled-rate freezer, which cools stem cells at the optimal rate of 1-2.5°C per minute (too slow a rate causes injury to the cells through solution effects; but at too high a rate intracellular freezing kills cells by rupturing cell membranes). Nevertheless, things can go wrong in the lab, and it is vital to know what to do to salvage cells if the controlled rate of freezing is interrupted for any reason.
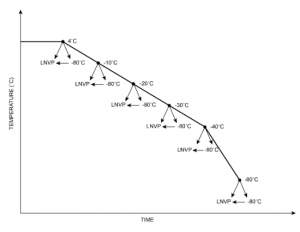
In our study we looked at what happens to cells when the freezing process is interrupted at different temperatures: 4°C, -10°C, -20°C, -30°C, -40°C and -80°C.
At each temperature, cells were removed from the freezer and either immediately immersed in vapour phase liquid nitrogen (LNVP) or stored for a few hours in a -80°C mechanical freezer before transfer to LNVP. After each treatment we thawed the test sample and assessed the viability of total nucleated cells (TNC), CD34+ cells, CD45+ cells, and colony-forming unit-grand macrophage (CFU-GM).
We used three calculations to determine the viability of the cells in the samples:
- percentage recovery of cells, calculated as the ratio of total cells in the treated sample divided by the unfrozen control;
- percentage viability of cells, calculated as the ratio of the viable CD45+ or CD34+ cells in the treated sample divided by the total number of living and dead cells in the treated sample; and
- percentage survival, calculated as the ratio of viable cells in the treated sample divided by the unfrozen control.
Our results show that post-thaw viability is not only affected by how the cells are treated and which assays are used to measure the health of the cells, but also by how success is defined.
We found that less than 5% of TNC and 10% of CD34+ cells were lost when the freezing process was interrupted at any temperature, regardless of whether the cells sample was transferred directly to LNVP or placed in a -80°C mechanical freezer before transfer to LNVP. However, the ability to count cells does not ensure that the cells being counted are alive.
We found that measuring the total number of cells (percentage recovery) is not a useful parameter for determining whether a sample subjected to an interruption in freezing is worth storing for later transplantation, because it includes dead as well as living cells, and other factors also generate misleading values.
The percentage viability of CD34+ cells, the assay that is used at most cryobanks, is unfortunately not accurate because it is affected by loss of dead cells (a smaller denominator in the ratio gives a false value for viability).
The percentage survival of CD34+ cells, as measured by the ratio of total viable cells to the total number of viable cells in the untreated sample, would seem to be the precise viability calculation to use. However, there was a complication even with this. When we assessed post-thaw survival using CFU-GM colonies, it was found to be significantly lower than that of CD34+ cells at all temperatures of interruption. Thus both the definition of success and the assay used to measure it are equally important in assessing post-thaw viability.
We concluded that the survival of CFU-GM should be used as the indicator of viability when cooling is interrupted, and that cells have to be transferred to LNVP at temperatures higher than -40°C.
We recommend that cord blood samples are transferred to a -80°C freezer before LNVP when a controlled rate freezer is not available or when freezing is interrupted. If direct transfer to LNVP is necessary, the cells should first have been cooled to -40°C or lower.
It would benefit the cord blood community if the viability assay was to be standardized, allowing cord blood banks and autologous stem cell programs to share and compare data reliably.
References:
- H.Yang, A. Pidgorna, M.R. Loutfy, P. Shuen. 2015. Effects of interruptions of controlled-rate freezing on the viability of umbilical cord blood stem cells. Transfusion 2015;55(1):70-76 doi: 10.1111/trf.12774