You are here
Vladomir’s Story: Vision Restored by Umbilical Cord Tissue MSC
 What is a mother ready to do, whose child is almost blind and unable to fully control his own body? After ten years of struggling with the consequences of brain damage that her child received because of trauma during childbirth, it seemed that Yuliya from Dnipro (central Ukraine) had tried everything. Finding that no available method worked, she decided to collect umbilical cord blood and umbilical cord tissue from the birth of her youngest child. During this time, she managed to learn about the opportunities offered by cell therapy, so after assessing all the risks and prospects, she decided to try it.
What is a mother ready to do, whose child is almost blind and unable to fully control his own body? After ten years of struggling with the consequences of brain damage that her child received because of trauma during childbirth, it seemed that Yuliya from Dnipro (central Ukraine) had tried everything. Finding that no available method worked, she decided to collect umbilical cord blood and umbilical cord tissue from the birth of her youngest child. During this time, she managed to learn about the opportunities offered by cell therapy, so after assessing all the risks and prospects, she decided to try it.
In 2008, Yuliya gave birth to twins at 32 weeks gestation. The children, Elizaveta and Vladomir, were born by caesarean section and each weighed a little over three pounds. Over time, the girl caught up with her peers and began to lead the life of an active, healthy child. But her brother Vladomir suffered Hypoxic-Ischemic Encephalopathy (HIE) brain damage at birth. At 1.5 years old, Vladomir was diagnosed with partial atrophy of the optic nerve in both eyes. Vladomir also had small seizures, as well as impairments of his coordination and fine motor skills. The doctors' verdict was that nothing can be done, the boy would never be able to see.
Hypoxic-Ischemic Encephalopathy occurs when a baby experiences oxygen deprivation at birth, and later these children are diagnosed as having Cerebral Palsy. The incidence of HIE varies between 1.5 to 10 per 1000 births, depending on the quality of healthcare in the country where the baby is born1. The original HIE injury triggers a cascade of brain damage that can lead to epilepsy, optic nerve atrophy, respiratory problems, or hydrocephalus2. The only therapeutic modalities that have been shown to benefit children with HIE are therapeutic hypothermia (cooling), if applied immediately after birth, or cell therapy, which also seems to be more beneficial if given sooner after the injury. Stem cell therapies for HIE have been studied in at least 19 preclinical animal studies, and over a dozen human clinical trials of cell therapy for HIE have been launched3-7. The largest body of evidence comes from studies of cell therapy for HIE with either the mononuclear stem cells from umbilical cord blood (CB-MNC) or the mesenchymal stromal stem cells from umbilical cord tissue (UC-MSC).
Vladomir’s parents did not rush to conceive a new child purely as a stem cell donor for their son. In the early years they struggled to take care of the twins, but as time passed they felt their family needed another baby. Yuliya said, “Like all pregnant women, I was afraid to be left alone with problems - where will I go with three children in my arms? In addition, when you are in your late 30s, you no longer have the desire to start all over again, to climb up again — you have a status, a job, a position, you are worried about losing everything. And the first children were born earlier - at 33 weeks, one child - with complications. There were a lot of things… But my husband supported me - he said: ‘Don't worry. We are writing a new story. We will be fine.’ And confidence in my husband – thanks to him - helped to go through all these hormonal surges, emotions, fears. And we really wrote a new story.”
In May 2019, Yuliya gave birth to a healthy baby boy. His umbilical cord blood and umbilical cord tissue were delivered to the Hemafund bank in Kyiv immediately after birth. Only 28 ml of umbilical cord blood was collected, but the immune type of the blood was compatible with Vladomir. The cord tissue was cultivated in the laboratory to produce umbilical cord MSC, which were then cryopreserved.
“I've known about stem cells for a long time, and of course, when I got pregnant, I couldn't help but take this opportunity. But I was looking for a company that would work in Ukraine.” It was necessary to choose a biobank that already had experience in obtaining MSCs from the umbilical cord, and that could provide the treatment in a medical clinic. Of all the banks represented in Ukraine, only Hemafund met the criteria.
Initially, Yuliya was afraid of the consequences of childbirth, and wanted to hold the cord blood and cord tissue in reserve as insurance for her new baby. But as it became clear that their youngest child was perfectly healthy, the family realized that these cells should go to Vladomir.
In July of 2019, Vladomir was infused with the cord blood of his baby brother. Six months later, 60 million cord tissue MSC were administered by three simultaneous pathways: by intravenous infusion, by intrathecal injection (into the spinal canal) and by retrobulbar injection (behind the occlusion of the optic nerve). In the United States, FDA-approved clinical trials have demonstrated that MSC injections into the eye can restore vision to patients with ophthalmologic conditions that were considered "untreatable", such as optic nerve atrophy8,9. Vladomir’s treatments were performed at Hemafund's clinic, QR-Health Solution. No adverse reactions were observed.
A medical follow-up at 12 months after the start of therapy showed notable improvements. The baseline electroencephalogram (EEG) of Vladomir’s brain had showed signs of seizure activity, mainly in the occipital lobes. He had decreased tendon reflexes and hyperreflexia of the arms and legs. However, by one year after the initial treatment, there were no signs of seizure activity on the EEG. Fine motor skills and coordination were improved. Vladomir is now active in sports; he plays football, wrestles, and rides a bicycle.
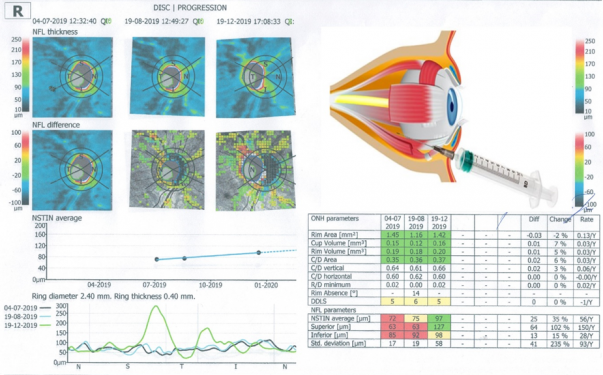
But the most dramatic, near-miraculous improvement, was in Vladomir’s vision. Prior to treatment, Vladomir’s visual acuity was 0.02 in both eyes. After treatment, his vision improved to 0.15 in the left eye and to 0.20 in the right eye. These measurements are taken on the decimal scale of visual acuity; on the Snellen scale that is used in the United States, visual acuity of 0.15 and 0.20 are equivalent to 20/133 and 20/100, respectively10. The accompanying figure displays optical coherence tomography of Vladomir’s right eye. The thickness of the retinal ganglion cells increased by 30% from the initial value. (Readers can click on this image to see it full size.)
The impact of improved vision on Vladomir’s life has been transformative. Where before he attended a special school for the blind, now he can read the second line of an eye chart and attends a regular school. His mother reports: “Vladomyr has started training, crossing the road - and I'm not afraid that he will be hit by a car. He rides a bicycle and scooter on his own, moves around the neighborhood. He recently climbed Hoverla (the highest mountain in the Carpathians) - and two years ago I couldn't even imagine that he would go somewhere by himself. The child did not see the moon in the sky, but now he sees the plane flying and can say its color! It gives me shivers…”
Vladomir's case is important because he has received significant benefits from stem cell therapy despite his age being adolescent at the time of treatment. Most of the clinical trials for HIE and Cerebral Palsy are aimed at treating young children. This case demonstrates the safety and efficacy of stem cell therapy for a child diagnosed with HIE accompanied by optic nerve atrophy. Intrathecal and retrobulbar administration of MSC allows the delivery of cell therapy directly to the affected tissues, in order to increase efficiency.
References
- Greco P, Nencini G, Piva I, Scioscia M, Volta CA, Spadaro S, Neri M, Bonaccorsi G, Greco F, Cocco I, Sorrentino F, D'Antonio F, Nappi L. Pathophysiology of hypoxic-ischemic encephalopathy: a review of the past and a view on the future. Acta Neurol Belg. 2020; 120(2):277-288.
- Nabetani M, Mukai T, Shintaku H. Preventing Brain Damage from Hypoxic-Ischemic Encephalopathy in Neonates: Update on Mesenchymal Stromal Cells and Umbilical Cord Blood Cells. American J Perinatology 2021; Epub ahead of print.
- Archambault J, Moreira A, McDaniel D, Winter L, Sun LZ, Hornsby P. Therapeutic potential of mesenchymal stromal cells for hypoxic ischemic encephalopathy: A systematic review and meta-analysis of preclinical studies. PloS ONE 2017; 12(12):e0189895.
- Liao Y, Cotten M, Tan S, Kurtzberg J, Cairo MS. Rescuing the neonatal brain from hypoxic injury with autologous cord blood. Nature BMT 2013; 48:890–900.
- Cotten CM, Murtha AP, Goldberg RN, Grotegut CA, Smith PB, Goldstein RF, Fisher KA, Gustafson KE, Waters-Pick B, Swamy GK, Rattray B, Tan S, Kurtzberg J. Feasibility of Autologous Cord Blood Cells for Infants with Hypoxic-Ischemic Encephalopathy. Pediatrics 2014; 164(5):973-979.e1
- Cotten CM, Fisher KA, Malcolm W, Gustafson K, Kurtzberg J. Phase II Clinical Trial of Autologous Cord Blood Cells for Neonates with Hypoxic-Ischemic Encephalopathy. Pediatric Academic Societies Abstract 2020
- Bruschettini M, Romantsik O, Moreira A, Ley D, Thébaud B. Stem cell-based interventions for the prevention of morbidity and mortality following hypoxic-ischaemic encephalopathy in newborn infants. Cochrane Database Systematic Reviews 2020; 8(8):CD013202.
- Weiss JN, Levy S. Autologous Bone-Marrow Derived Stem Cells in the Treatment of “Untreatable” Optic Nerve and Retinal Conditions. EC Ophthalmology 2018; 9(5):332-336.
- Weiss JN, Levy S. Stem Cell Ophthalmology Treatment Study (SCOTS): bone marrow derived stem cells in the treatment of Dominant Optic Atrophy. Stem Cell Investig. 2019; 6:41.
- NIDEK. Conversion Table for Representation of Visual Acuity. Accessed 2021-12-01