You are here
Umbilical Cord Tissue Cells: Challenges and Promise
 Today, among expectant parents who choose to bank cord blood in Asia, Europe and North America, 57% also have the option to store umbilical cord tissue, or the cells derived therefrom.1
Today, among expectant parents who choose to bank cord blood in Asia, Europe and North America, 57% also have the option to store umbilical cord tissue, or the cells derived therefrom.1
What concerns me is that this percentage is not considerably higher—and that there is, currently, only one public repository for Wharton’s Jelly mesenchymal stromal (stem) cells in the western hemisphere.
As mentioned by Pedro Silva Couto in a previous edition of this Newsletter, “Parents who wish to store MSC from cord tissue should look at exactly what kind of services are being offered by the stem cell banks, how they perform the quality control tests, and ultimately what kind of guarantees they offer in case of the sample is released for transplantation purposes.”2
But there are two glaring problems in this otherwise perfectly sage advice. First, it assumes that expectant parents, or grandparents, are sufficiently knowledgeable to judge the veracity of claims and processes described by cell banks: but this is of very low probability. Second, and of equally low probability, it assumes that parents would be able to evaluate the “guarantees” offered by the companies in question.
Those of us involved with the harvesting of cord tissue and conducting research—basic or clinical—with the cells derived therefrom should rise to the challenge of providing clear and consistent information that can aid not only parents, but also the physicians who advise them.
Certain facts about the umbilical cord have become conventional wisdom: We know that the umbilical cord is a rich source of MSCs. We know that perinatal tissues are a better source of cells than adult tissues because their cell senescence is delayed and cell expansion is expedited.
Yet there is a disturbing lack of consensus on the anatomical descriptors of the cord tissue. Moreover, both academic and commercial descriptions of methods to isolate cells often lack sufficient transparency to be easily reproduced. These problems combine to hamper scientific progress within the field, as well as make it difficult for cell banking companies to clearly communicate their services so that parents can make fully informed decisions.
Below I will address 3 topics:
- What is the structure of the umbilical cord and what cells can be harvested?
- What cells are being used in current cell therapies employing umbilical cord tissue? and
- What are the diseases treated with umbilical cord cells today?
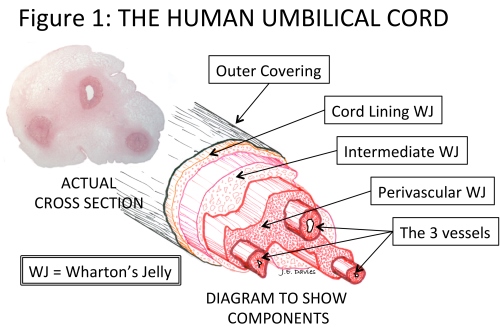
The umbilical cord, of course, attaches the mother (placenta) to the developing baby (fetus). This rope-like structure has a very thin outer covering and contains three blood vessels that transport nutrients to/from the fetus from/to the placenta. As pregnancy progresses, and the baby grows, a healthy and adequate blood supply is vitally important. The umbilical cord grows rapidly in both length and girth to accommodate this essential transport of nutrients. To support the blood vessels, and prevent them kinking as the baby starts to move in the womb, they are surrounded by a special, and unique, cushioning connective tissue called Wharton’s Jelly (WJ) - see Figure 1.3
The problem with this apparently simple story is that WJ has been described in a variety of ways by different authors. For example, the term is sometimes used to describe all the cells isolated by enzymatic digestion from the cord tissue. Wharton’s Jelly has also been described as consisting of six different regions.4 Only isolation of cells from the umbilical cord lining, and from the WJ of the perivascular zone, have been described in sufficient detail—and are the subject of many patents—to enable isolation of cells from these regions to the exclusion of others. However, descriptions of the size of the perivascular zone range from 2 cells thick5, to the substantial structure illustrated in Figure 1.
Such disparities in the descriptions of cord tissue clearly provide a barrier to not only understanding the differences in services offered by cord tissue banking companies, but also the interpretation of data provided by either basic scientific or clinical studies. This has been explained in greater detail elsewhere as a foundation for a consensus description of human umbilical cord structure.6 If those of us in the profession can arrive at a common understanding and communicate it clearly, it will be far easier for parents to make the important decisions they face.
 The value of cord tissue lies in the cells contained within the extracellular matrix. The tissue itself has not been shown to have any therapeutic value; but averaged over the past five years cord tissue now represents the fastest growing source of MSCs for human clinical trials.7
The value of cord tissue lies in the cells contained within the extracellular matrix. The tissue itself has not been shown to have any therapeutic value; but averaged over the past five years cord tissue now represents the fastest growing source of MSCs for human clinical trials.7
Of course, there are many types of cells in cord tissue—the amniotic epithelium, the cells of Wharton’s Jelly, endothelial cells from the vessel lining; or smooth muscle cells from the walls of the three vessels. Of these, it is only Wharton’s Jelly that has been shown by numerous studies to be a rich source of MSCs—and it is only MSCs that are the focus of all the “cord tissue” clinical trials today.7 Indeed, MSCs are the only stem cells to have been found in umbilical cord tissue. The clinical value of cord tissue therefore rests within the WJ cells (see Side Panel 1). The obvious question to ask would be: Where do you find these important cells in Wharton’s Jelly?
The answer is quite simple. Generally, MSCs are found throughout the adult body–but they are always found near blood vessels—and the umbilical cord is no exception. So the vast majority of MSCs in the human umbilical cord is found around the three blood vessels of the cord in the so-called perivascular region (see Figure 1 and Side Panel 2) and can differentiate to become the functional cells in the jelly (myofibroblasts).8
 But to be of real therapeutic value cells are commonly employed in large numbers. For example, to treat a systemic disease hundreds of millions of cells are usually required. This means that the cells harvested from Wharton’s Jelly have to be expanded in number to produce a therapeutic dose. This can be illustrated in the following example: The total number of cells in the umbilical cord has been calculated to be about 11 million cells/gram of umbilical cord.9 Of this total number of cells almost 80% make up the vessels of the cord; and the remaining 20% are in Wharton’s Jelly. A typical umbilical cord harvested at the birthing site and sent to a family bank, would yield about 35 million Wharton’s Jelly cells — sometimes described as a “treatment ready dose”—but which is less than the clinical dose to inject into one adult osteoarthritic knee.10 Cell expansion is thus essential for the vast majority of applications of MSCs.
But to be of real therapeutic value cells are commonly employed in large numbers. For example, to treat a systemic disease hundreds of millions of cells are usually required. This means that the cells harvested from Wharton’s Jelly have to be expanded in number to produce a therapeutic dose. This can be illustrated in the following example: The total number of cells in the umbilical cord has been calculated to be about 11 million cells/gram of umbilical cord.9 Of this total number of cells almost 80% make up the vessels of the cord; and the remaining 20% are in Wharton’s Jelly. A typical umbilical cord harvested at the birthing site and sent to a family bank, would yield about 35 million Wharton’s Jelly cells — sometimes described as a “treatment ready dose”—but which is less than the clinical dose to inject into one adult osteoarthritic knee.10 Cell expansion is thus essential for the vast majority of applications of MSCs.
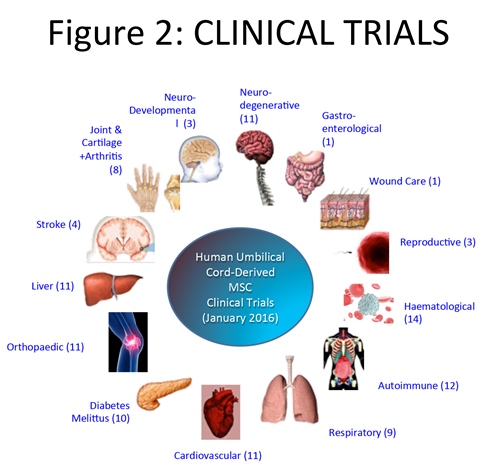
The first clinical trial using MSC from cord tissue was in 2008, and through the end of 2015 there have been 95 trials worldwide for the treatment of a plethora of indications.7 Most of these studies focus on the important effects these fascinating cells have on the immune system. Most parents today know that the best place to find international details on current clinical trials is the US government site, ClinicalTrials.gov. To find the results of clinical trials it is necessary to search the medical literature. Published studies show that umbilical cord tissue cells can be employed safely in the clinic, and for some indications may have beneficial therapeutic effects.
As is often the case with basic science studies, the published clinical reports used a variety of cell isolation procedures, which abrogate comparative assessment of the therapeutic benefits claimed. More transparency is required in the literature describing the cell populations isolated, and this should be based upon a community consensus on the anatomical structure of the cord and, particularly, the contained Wharton’s Jelly.
 Despite these hurdles, I am confident that great therapeutic value can be gained from the MSCs in Wharton’s Jelly, which are being employed in an ever-increasing portfolio of indications. The latter include the use of genetically modified WJ cells as delivery vehicles for monoclonal antibodies11, the co-administration of WJ cells as a prelude, or complement, to organ transplant, and medical countermeasures to bioweapon exposure—all of which may be covered in a follow-up in this Newsletter.
Despite these hurdles, I am confident that great therapeutic value can be gained from the MSCs in Wharton’s Jelly, which are being employed in an ever-increasing portfolio of indications. The latter include the use of genetically modified WJ cells as delivery vehicles for monoclonal antibodies11, the co-administration of WJ cells as a prelude, or complement, to organ transplant, and medical countermeasures to bioweapon exposure—all of which may be covered in a follow-up in this Newsletter.
References
- Cord Blood Industry Report 2015; CordBloodIndustryReport.org
- Couto PS May 2014; Storage of Mesenchymal Stem/Stromal cells in family stem cell banks: What do they offer?
- Tissue Regeneration Therapeutics Inc. www.VeryPowerfulBiology.com
- Can A, Karahuseyinoglu S. 2007; Concise Review: Human Umbilical Cord Stroma with Regard to the Source of Fetus-Derived Stem Cells. Stem Cells 25:2886–2895.
- Kita K, et al. 2010; Isolation and Characterization of Mesenchymal Stem Cells From the Sub-Amniotic Human Umbilical Cord Lining Membrane. Stem Cells Dev. 19(4):491–502.
- Davies JE et al 2017; Mini-review. Stem Cells Translational Medicine. In Press
- Cell Trials Data CellTrials.org
- Nanaev AK, Kohnen G, Milovanov AP, et al. 1997; Stromal differentiation and architecture of the human umbilical cord. Placenta 1997;18:53–64.
- Schugar RC et al. 2009; High harvest yield, high expansion, and phenotype stability of CD146 mesenchymal stromal cells from whole primitive human umbilical cord tissue. J. Biomed. Biotechnol. 2009:789526.
- Centeno C et al. 2015; A dose response analysis of a specific bone marrow concentrate treatment protocol for knee osteoarthritis. BMC Musculoskeletal Disorders 16:258
- Braid LR et al. 2016; Engineered Mesenchymal Cells Improve Passive Immune Protection Against Lethal Venezuelan Equine Encephalitis Virus Exposure. Stem Cells Trans Med doi: 10.5966/sctm.2015-0341


