You are here
TotiCyteTM - the next generation of cord blood processing technology
The most important factors in determining a successful stem cell transplant are human leukocyte antigen (HLA) match and number of living (viable) cells available for treatment.
HLA is the finger print equivalent to see if the donor matches the patient. If they do not, then game over. However, if there is a good enough match, then 25 million total nucleated cells (TNC) per kg of bodyweight are required to give the patient the best chance of a successful stem cell transplant.
Many public cord blood banks apply a very high TNC threshold before they will store a cord blood unit for possible transplant use. Thresholds between 140 and 200 billion TNC increase the probability that the samples they process will be therapeutically useful.
But few babies have a large enough volume of cord blood to reach this threshold. In the end, 80-90% of cord blood donations go to waste.
Volume reduction technologies
The leading methods of processng cord blood for storage employ a volume reduction step that aims to remove some of the non-useful red blood cell fraction and the plasma. For example, a starting volume of 100ml will be reduced to 25ml for final storage.
However, all volume reduction technologies result in stem cell losses approaching up to 40% (1), and in some instances, the almost total loss of potentially important low abundance cells (2). Significant levels of red blood cells remain, which can be up to 30% of the starting concentration (3).
TotiCyteTM - a new paradigm in cell recovery
Cells4Life has developed TotiCyteTM, a processing method which selectively removes the red blood cells in cord blood and yields:
- over 95% recovery of the white cell fraction,
- 99.5% removal of the red cells,
- obviates the need for washing to remove DMSO and red cells, and
- exceptionally high post thaw viable cell recovery
TotiCyteTM is a mixture of compounds already commonly used in blood product processing. When added to cord blood, gravity causes the red cells to selectively sediment within 30 minutes and the white cells remain in solution. These white cells can be easily separated from the sedimented red cells, and the important white cells can then be concentrated by gentle centrifugation into a small volume containing less than 0.5% of the original red cell content. Addition of a small amount of DMSO for cryogenic storage and controlled freezing using standard procedures then completes this simple process.
Post-thaw recovery
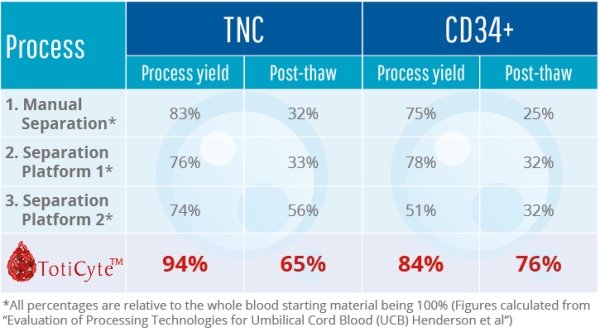
One of the most exciting features of TotiCyteTM is improved post-thaw recovery. When cord blood stem cells are cryopreserved and then thawed for therapy, large cell losses occur during thawing. With TotiCyteTM, the viable cell recovery post-thaw is markedly increased. Approximately 76% of the CD34+ remains viable, whereas post-thaw viability with the current leading separation methodology can be as low as 32% in some instances (4).
Entire white cell fraction
TotiCyteTM allows almost the entire white cell fraction to be isolated and/or concentrated, with only modest, if any, loss of any cell type thus far examined. Other processing methods frequently result in the loss of the megakaryocyte-like (CD45+CD61+) cells and/or MNC-like (CD45+CD34+) cells.
After processing with TotiCyteTM, we have identified a significant population of cells that express both Oct4 and Nanog, indicating that very early progenitor cells are maintained by our method. By comparison, the so-called Very Small Embryonic Like stem cells (VSELs) are lost in processing methods that employ centrifugal first stage separation (2).
Impact for the public banking model
TotiCyteTM will positively impact the value chain of public cord blood banks in several ways:
In summary, our results show that TotiCyteTM can deliver a larger number of viable cells for therapeutic use and more stem cell types than any other volume reduction technology.
The public banking business model suffers from high wastage of donations and under-distribution of stored cord blood. TotiCyteTM will increase public banking profitability both by reducing the number of donations that are discarded and by increasing the quality / marketability of the stored cord blood units.
References:
- Lapierre, V., Pellegrini, N.,Bardey, I., Malugani, C., Saas, P., Garnach, F., Racadot, E., Maddens, S., & Schillinger, F. Cord blood volume reduction using an automated system (Sepax) vs. a semi-automated system (Optipress II) and a manual method (hydroxyethyl starch sedimentation) for routine cord blood banking: a comparative study. Cytotherapy. 2007; 9(2):165-9.
- Bhartiya, D., Shaikh, A., Nagvenkar, P., Kasiviswanathan, S., Pethe, P., Pawani, H., Mohanty, S., Rao, A., Zaveri, K. & Hinduja, I. Very Small Embryonic-Like Stem Cells with Maximum Regenerative Potential Get Discarded During Cord Blood Banking and Bone Marrow Processing for Autologous Stem Cell Therapy. Stem Cells and Development. 2012; 21(1):1-6.
- Basford, C., Forraz, N., Habibollah, S., Hanger, K. & McGuckin, C. The Cord Blood Separation League Table: a Comparison of the Major Clinical Grade Harvesting Techniques for Cord Blood Stem Cells. International Journal of Stem Cells. 2010; 3(1):32-45.
- Henderson, C., Wofford, J., Fortune, K. & Regan, D. Evaluation of Processing Technologies for Umbilical Cord Blood. 2010; ISCT Poster annual meeting, Philadelphia, USA.


