You are here
Macopharma cord blood collection bag now available in the United States
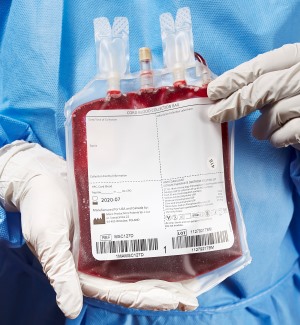
Macopharma has now launched their dual needle, FDA NDA approved cord blood collection bag in the United States. Macopharma has been a leading supplier of cord blood collection kits in Europe and across the rest of the world for over 15 years.
The Macopharma collection kits include a variety of innovative features. These include an additional 12G needle, graduations to provide an indication of the collected blood volume, and a CPD rinsing pouch. The exterior of the bag is sterile and has an overwrap making the product suitable for caesarian section deliveries.
It is hoped that the launch of the kit will provide some much-needed options to US cord blood banks looking to optimize their collection procedures. Macopharma’s aim is to assist banks in the common goal of improving the number of bankable cord blood units collected in the United States.
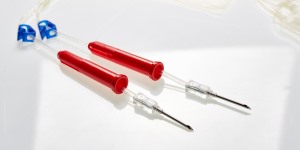
PRODUCT FEATURES
• Red permanent clamp
• Graduations showing volume indication
• Sterile inside inner overwrap - suitable for C-Section deliveries
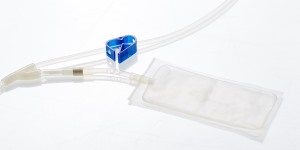 SAFETY AND PERFORMANCE
SAFETY AND PERFORMANCE
• Maximum collection volume of up to 250 mL
• 35 mL of Citrate Phosphate Dextrose (CPD) anticoagulant solution (including 8 mL in rinsing bag)
• In-line rinsing bag containing anticoagulant allows for maximal volume recovery frm the tubing
• 12-gauge needles to aid in maximizing flow
• The double needle system enables users to make a second venipuncture to assist in maximizing volume recovery
• SecuvamTM needle guard minimizes the potential risk of accidental needle stick injury
• 2 year shelf life



 Macopharma designs innovative solutions and quality products that meet the needs of donors, patients, and healthcare providers. Macopharma is a world leader in offering blood transfusion products and services to collect, process, and store blood components that meet high safety and quality standards for patient transfusion.
Macopharma designs innovative solutions and quality products that meet the needs of donors, patients, and healthcare providers. Macopharma is a world leader in offering blood transfusion products and services to collect, process, and store blood components that meet high safety and quality standards for patient transfusion.