You are here
Immune Cell Banking
Personal immune cell banking may be an idea whose time has come. Within the cell therapy industry, there is some controversy surrounding the feasibility and advisability of immune cell banking for personal use. In this article we explain the applications for immune cells, estimate the likelihood of using stored immune cells, describe how immune cell banking has parallels to cord blood banking, and we explore the technical and regulatory hurdles facing the nascent industry of immune cell banking.
What is immunotherapy, especially CAR-T?
Immunotherapy is a field of medicine that is growing rapidly, and this forms the motivation for immune cell banking. The definition of Immunotherapy includes any therapies that stimulate or suppress the immune system. Recently there has been a great deal of excitement in the oncology field over breakthrough therapies that fight cancer by harnessing specific immune cells, such as T-cells or NK cells.
CAR-T therapy is based on Chimeric Antigen Receptor (CAR) T-cells1. The patient’s T-cells, a type of cell in the immune system, are collected and sent to a lab where they are genetically modified to have chimeric antigen receptors on the cell surfaces. The receptors are designed to recognize a target antigen on the patient’s cancer. These modified cells are then expanded in number, cryopreserved, and sent back to the patient’s hospital. When the CAR-T cells are infused into the patient, they attack the cancer cells that have the target antigen. This attack may be so successful in rapidly killing large numbers of cancer cells that the patient may become sick with cytokine release syndrome2,3. During the decade from 2010 to 2020, the number of CAR-immunotherapy clinical trials grew 36-fold4.
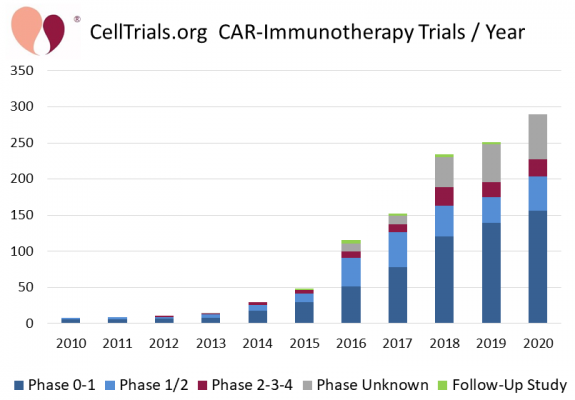 From 2017 until now (1 April 2021), five CAR-T therapies have been approved by the FDA, and they are all autologous therapies that use the patient’s own cells (the product names are Kymriah, Yescarta, Tecartus, Breyanzi, Abecma)5-9. The early clinical trials of CAR-T therapies achieved high response rates for several hematologic cancers3. Also, the early clinical trials of CAR-T therapies only enrolled high-risk cancer patients that were heavily pre-treated and had failed multiple chemotherapies, including stem cell transplants10-12. Those CAR-T products approved by the FDA have transitioned from the strict criteria in clinical trials to being prescribed more liberally by treating physicians13-15.
From 2017 until now (1 April 2021), five CAR-T therapies have been approved by the FDA, and they are all autologous therapies that use the patient’s own cells (the product names are Kymriah, Yescarta, Tecartus, Breyanzi, Abecma)5-9. The early clinical trials of CAR-T therapies achieved high response rates for several hematologic cancers3. Also, the early clinical trials of CAR-T therapies only enrolled high-risk cancer patients that were heavily pre-treated and had failed multiple chemotherapies, including stem cell transplants10-12. Those CAR-T products approved by the FDA have transitioned from the strict criteria in clinical trials to being prescribed more liberally by treating physicians13-15.
The industry of cell and gene therapy is improving the efficiency of CAR-T manufacturing, creating later generations of CAR-T products, and broadening the applications of CAR-T therapy16-24. Many scientists and clinicians are hopeful that CAR-T therapy will be applied to more cancers, earlier in the course of treatment, and become a routine tool for oncologists. It should be noted that the more immunotherapy is successful at treating cancer patients, the less need there will be for stem cell transplants. The success of the immunotherapy industry may come at the expense of the organizations that provide grafts and services for stem cell transplants.
What is immune cell banking?
The basic concept of immune cell banking is to isolate white blood cells, including T-cells as well as other immune cells, from peripheral blood, for potential future use. In order to be useful for immunotherapy, the immune cells should be collected with high purity. Some immunotherapy products require starting with a large number of cells as well. Once collected, the immune cells can then be cryopreserved by standard methods.
A few companies are starting to offer personal immune cell banking as a consumer service. The personal biobanking of immune cells is a form of medical insurance, against the possibility that the client will someday need a therapy based on immune cells. It is the nature of all insurance policies to be a speculative service, spending money today for protection against the odds that something unfortunate will happen later.
What are the odds of a diagnosis that could use personal immune cells?
Today, CAR-T therapies are only given to patients that have failed other cancer therapies10-15. Companies developing CAR-T therapies hope that in the future immunotherapy will become a frontline therapy for these patients. As a starting point to calculate the odds of use, we assume that the likelihood of a person receiving CAR-T therapy is comparable to the likelihood of having a stem cell transplant. Based on public health data from 2001-2003, the cumulative odds of a US citizen having a stem cell transplant by age 70, either autologous or allogeneic (with a donor always available), were 1 in 21725. Bear in mind those numbers are based on patients that actually had a stem cell transplant. The number of potential CAR-T patients would be higher if we included half of all patients diagnosed with a transplantable illness, which was about 32 thousand US patients per year in 2001-200325.
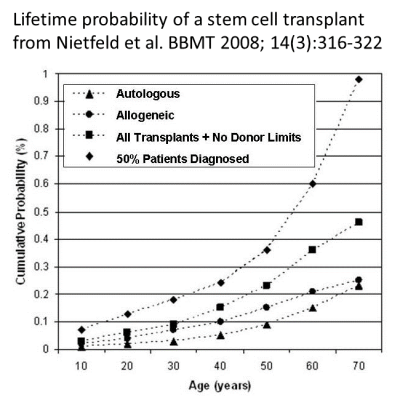 Another paper published by cell therapy experts in 2019 estimated the annual number of patients receiving CAR-T therapy by taking all patients diagnosed with blood cancers and assuming that about one third of them would receive CAR-T therapy. This led the authors to an estimate of 18 thousand CAR-T patients per year in the US19. Bear in mind they only looked at blood cancers, whereas the previous estimate included all transplantable conditions, so these two estimates are compatible within reasonable margins of error19,25.
Another paper published by cell therapy experts in 2019 estimated the annual number of patients receiving CAR-T therapy by taking all patients diagnosed with blood cancers and assuming that about one third of them would receive CAR-T therapy. This led the authors to an estimate of 18 thousand CAR-T patients per year in the US19. Bear in mind they only looked at blood cancers, whereas the previous estimate included all transplantable conditions, so these two estimates are compatible within reasonable margins of error19,25.
These forecasts all have large uncertainties because the indications for using CAR-T are evolving22-24. Plus, our forecast has focused simply on oncology applications and has not included the potential use of CAR-T therapy for non-malignant diseases, such as autoimmune disorders26.
What is the future of personal (autologous) CAR-T therapy?
There are two camps of opinion regarding the future of autologous CAR-T therapy derived from the patient’s own cells: one side thinks it is a growth opportunity, and the other side thinks it is doomed.
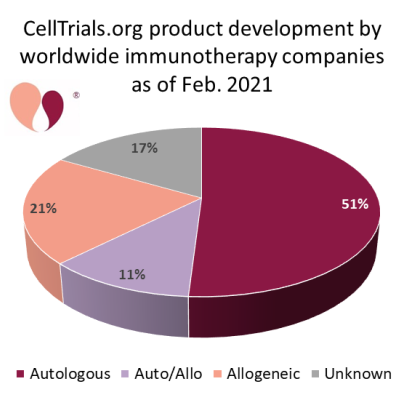 On the one hand, we can safely bet that over the next decade autologous CAR-T therapies will become more readily available to patients. They should become easier to manufacture and distribute efficiently, be applied for more indications, and be offered earlier in the course of treatment instead of after multiple failures16-24.
On the one hand, we can safely bet that over the next decade autologous CAR-T therapies will become more readily available to patients. They should become easier to manufacture and distribute efficiently, be applied for more indications, and be offered earlier in the course of treatment instead of after multiple failures16-24.
On the flip side, a growing number of pharmaceutical companies are working to develop allogeneic CAR-T therapies that do not rely on the patient’s own cells. The resource CellTrials.org maintains a database of companies that are developing immunotherapy: between Feb. 2020 and Feb. 2021 the number of companies worldwide grew from 357 to 451, and 32% of these companies are currently known to be developing allogeneic products27,28. Some professionals in the cell therapy field believe that eventually allogeneic CAR-T will replace autologous CAR-T, that it is only a matter of time until that happens. Other professionals believe that autologous CAR-T will always have a niche. New technologies can easily change the balance of this competition. For example, one company has announced a technology to produce autologous CAR-T with next-day manufacturing29. No one has a crystal ball to read the future and know how the balance between autologous and allogeneic CAR-T therapies will stabilize in the long term.
Analogy between immune cell banking and cord blood banking
When immune cell banking is offered to healthy individuals as biological insurance, the business model is very similar to private cord blood banks, where families decide to bank their newborn’s stem cells to protect their future health. In the beginning, family cord blood banking was very speculative: The first cord blood transplant was performed in 198830, the first public cord blood bank was launched in 199231, and the first family bank followed swiftly thereafter32. Over the first decade of family cord blood banking, from 1995 to 2005, the only motivation for banking cord blood was in case a child had pediatric cancer, where the cumulative odds of a stem cell transplant by age 20 are only 3 in 5,00025.
But in 2005, something happened which changed the potential applications of cord blood in family banks. That was the year that Dr. Kurtzberg’s group at Duke University started to use autologous cord blood stem cells to treat pediatric neurologic conditions33. It was not until about a decade later that several groups published controlled studies describing the use of cord blood for cerebral palsy (CP), and later for autism spectrum disorder (ASD)34-39. In 2017 the FDA approved an expanded access protocol NCT03327467 that allows cord blood infusions for children with brain injuries (allowed donors are both autologous and siblings). Since then, the majority of releases from family cord blood banks are to treat children with CP and ASD. In Feb. 2021, Duke University received a patent on the use of autologous cord blood for ASD40. The prevalence of CP is 1 in 345 among school-age children in the United States, and the prevalence of ASD is 1 in 54 children41,42.
The experience of family cord blood banks offers a cautionary tale for immune cell banks on several levels. The first caution is that any form of speculative banking tends to be met with resistance43. When autologous cord blood was adopted for treating neurologic conditions, the odds of using units stored in family banks increased by over a factor of 30. But, a second caution is that it took more than two decades from the launch of the first family cord blood bank, before these neurologic indications for use became widely adopted. Third, the final caution is that when the industry was launched, no one could foresee that neurologic applications of cord blood would completely change the landscape.
It is reasonable to say that no one today can predict where banked immune cells may be applied even a decade from now, let alone a quarter of a century from now.
Logistics of immune cell banking in the process of manufacturing immunotherapies
Speculative banking from healthy individuals is only one business model for immune cell banks. Another, more pragmatic model, is to offer immune cell banking to cancer patients at the time of diagnosis. This will give the patients access to relatively fresh autologous cells, before their immune systems have been damaged by chemotherapy, in case they need some form of immunotherapy later. Systematic banking of immune cells from cancer patients is beyond the laboratory capacity of many cancer treatment centers, and would require partnerships with third party immune cell banks.
The process of manufacturing a CAR-T therapy from a patient’s own cells usually starts with apheresis to harvest white blood cells from the immune system, including T-cells. Numerous publications have described the logistics of manufacturing autologous CAR-T therapies and how they might be optimized16-21. The immune cells are shipped to a centralized manufacturing facility where the T-cells are converted into a CAR product under cGMP conditions. The final step is to ship the cryopreserved CAR product back to a treatment site where it is thawed and administered to the patient. Some companies feel that there is less risk and more efficiency if the patient’s apheresis harvest is cryopreserved before shipping the cells to the manufacturing facility17,21. There is no fundamental reason why the starting cryopreserved white blood cells can’t be supplied by an immune cell bank, provided the bank follows validated procedures and has proper accreditations.
 Studies have shown that the quality of a patient’s immune system at the time of starting CAR-T therapy plays a role in the outcome, but more investigation is needed to fully explore the impact. We can illustrate this by looking at Yescarta (generic name Axicabtagene Ciloleucel), a CAR-T therapy from Kite, a Gilead company, that was approved for diffuse large B-cell lymphoma (DLBCL)6. The Zuma-1 clinical trial that led to the approval of Yescarta had very strict eligibility criteria, and at many cancer centers roughly half of the patients with DLBCL would not qualify for the trial13-15. The primary reasons for patients being rejected were aggressive disease or low blood counts. Once Yescarta had FDA approval, physicians could prescribe it to patients that did not meet the trial criteria, and could give bridge therapies while it was being manufactured. Retrospective studies have found that real world patient survival has been comparable for patients who would or would not have been eligible for the trial, although trial-worthy patients fared somewhat better14,15. More study is needed to explore a suggested relationship between white blood cell count at the time of apheresis versus response14. To date, there is no research comparing patient survival after standard CAR-T therapy versus therapy based on previously banked immune cells.
Studies have shown that the quality of a patient’s immune system at the time of starting CAR-T therapy plays a role in the outcome, but more investigation is needed to fully explore the impact. We can illustrate this by looking at Yescarta (generic name Axicabtagene Ciloleucel), a CAR-T therapy from Kite, a Gilead company, that was approved for diffuse large B-cell lymphoma (DLBCL)6. The Zuma-1 clinical trial that led to the approval of Yescarta had very strict eligibility criteria, and at many cancer centers roughly half of the patients with DLBCL would not qualify for the trial13-15. The primary reasons for patients being rejected were aggressive disease or low blood counts. Once Yescarta had FDA approval, physicians could prescribe it to patients that did not meet the trial criteria, and could give bridge therapies while it was being manufactured. Retrospective studies have found that real world patient survival has been comparable for patients who would or would not have been eligible for the trial, although trial-worthy patients fared somewhat better14,15. More study is needed to explore a suggested relationship between white blood cell count at the time of apheresis versus response14. To date, there is no research comparing patient survival after standard CAR-T therapy versus therapy based on previously banked immune cells.
We can illustrate another patient challenge by looking at Kymriah (generic name Tisagenlecleucel), the first approved CAR-T therapy from Novartis5. The guidelines for manufacturing this product require a starting dose of CD3+ T-cells that is at least 0.6 billion, with a target of 2 billion44-46. Some cancer patients have trouble providing enough T-cells to manufacture this product. The patients must stop chemotherapy at least 2 weeks before apheresis, and be at least 3 months out from an allogeneic stem cell transplant. As the authors of one paper say, “This represents a fragile balance between good quality and quantity of T effector cells and the urge to treat a refractory patient”45.
Some of the challenges that prevent cancer patients from accessing CAR-T therapy today could be removed if those patients had routinely banked their immune cells in advance, in case of later need for immunotherapy.
Immune cell banking and the ISCT
The International Society for Cell and Gene Therapy (ISCT) is a professional society for both scientists and clinicians that work in cell therapy. It is very common for professional societies, especially those in medical fields, to adopt a code of ethics for their members. In the case of the ISCT, the membership pledge requires members to read and accept their position statements on “unproven cellular therapies”47-49.
In addition, the ISCT has gone a step further and issued statements saying that the business of commercial cell banking is not supported by the “current scientific evidence”43,50,51. In 2019 the ISCT convinced some of the other organizations that work in cell therapy to endorse this position51. The opposition from ISCT leadership is the primary reason that immune cell banking is controversial. But is it realistic for a professional society to attempt to limit business models in a field where the technology is rapidly evolving?
The ISCT opposition to personal immune cell banking rests on two pillars: one, the fact that it is speculative banking, and two, the argument that what immune cell banks store is not useful for therapy. The first point will always be valid, because the motivation for any form of insurance policy, including immune cell banking, will always rest on speculation. However, the second argument rests on a series of technical issues which can all be removed by advancements in banking technology.
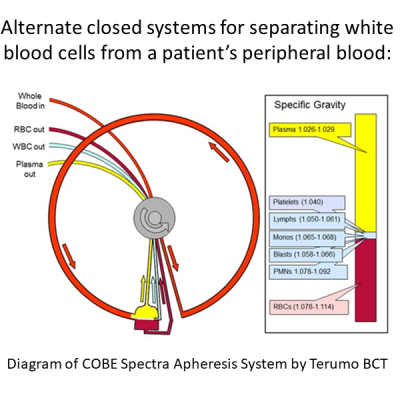
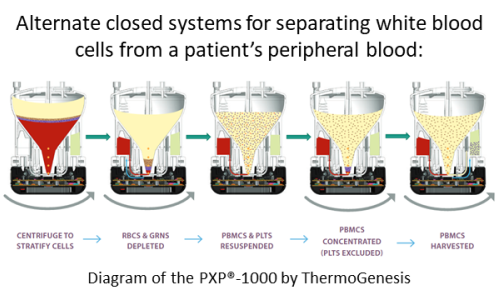 For example, it has been argued that immune cell banks do not store “enough cells” to be clinically useful for manufacturing a CAR-T therapy43. The number of cells that are enough to start manufacturing varies from one product to another. The traditional approach to banking cells is to take a 50ml blood draw and process it with the manual Ficoll technique52. But if the bank processes 200ml of blood in a closed cGMP medical device, the objection of not having enough cells can probably be removed53,54. This is just one example, but any technological advances that allow banks to store larger cell doses and/or allow CAR-T manufacturing to start from smaller cell doses will remove the claim that immune cell banks don’t have enough cells20,46.
For example, it has been argued that immune cell banks do not store “enough cells” to be clinically useful for manufacturing a CAR-T therapy43. The number of cells that are enough to start manufacturing varies from one product to another. The traditional approach to banking cells is to take a 50ml blood draw and process it with the manual Ficoll technique52. But if the bank processes 200ml of blood in a closed cGMP medical device, the objection of not having enough cells can probably be removed53,54. This is just one example, but any technological advances that allow banks to store larger cell doses and/or allow CAR-T manufacturing to start from smaller cell doses will remove the claim that immune cell banks don’t have enough cells20,46.
Another concern is that regulatory agencies require that the manufacture of CAR-T products must follow current Good Manufacturing Practices16,43. The chain of cGMP compliance goes all the way back to the procurement of raw materials. So far, CAR-T clinical trials that run at multiple sites, perhaps in multiple nations, have accepted patient cells from multiple apheresis clinics that have differences in equipment, procedures, and staff training16-21. Going forward, it is desirable to train participating apheresis clinics to keep the records required for cGMP products55. If immune cells from personal banks are to be used for product manufacture, they need to comply with this regulatory framework. But again, this is a technical hurdle and not insurmountable.
Summary
Immunotherapy is a rapidly evolving field, where both the technologies used to create products and the indications for use of products are subject to change. Many professionals expect that within a few years autologous CAR-T will be more readily available to more patients. Some of the challenges that currently prevent cancer patients from accessing CAR-T therapy could be removed if those patients had routinely banked their immune cells in advance, in case of later need for immunotherapy. Personal immune cell banking may be an idea whose time has come.
References
- Leukemia & Lymphoma Society. Chimeric Antigen Receptor (CAR) T-Cell Therapy. LLS website Accessed 2021-03
- Rosenbaum L. Tragedy, Perseverance, and Chance — The Story of CAR-T Therapy. NEJM 2017; 377(14):1313-1315.
- June CH, Sadelain M. Chimeric antigen receptor therapy. NEJM 2018; 379(1):64-73.
- Bersenev A, Verter F. Immunotherapy Clinical Trials in 2019. CellTrials.org News Published 2020-02-22
- FDA. FDA approval brings first gene therapy to the United States. FDA News Release Published 2017-08-30
- FDA. FDA approves CAR-T cell therapy to treat adults with certain types of large B-cell lymphoma. FDA News Release Published 2017-10-18
- FDA. FDA Approves First Cell-Based Gene Therapy For Adult Patients with Relapsed or Refractory MCL. FDA News Release Published 2020-07-24
- FDA. FDA Approves New Treatment For Adults With Relapsed Or Refractory Large-B-Cell Lymphoma. FDA News Release Published 2021-02-05
- FDA. FDA Approves First Cell-Based Gene Therapy for Adult Patients with Multiple Myeloma. FDA News Release Published 2021-03-27
- Neelapu SS, Locke FL, Bartlett NL, M.D., et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. NEJM 2017; 377:2531-2544.
- Schuster SJ, Bishop MR, Tam CS, et al., for the JULIET Investigators. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. NEJM 2019; 380:45-56.
- Schuster SJ, Bishop MR, Tam CS, et al. Long-Term Follow-up of Tisagenlecleucel in Adult Patients with Relapsed or Refractory Diffuse Large B-Cell Lymphoma: Updated Analysis of Juliet Study. TCT 2019; 25(3S):S20-S21.
- Reddy P, Sokolova A, Chow VA, ... Smith SD. Eligibility for Car-T Trials: An Analysis of Selection Criteria and Survival Outcomes in Chemorefractory DLBCL. Blood 2018; 132(S1):2911.
- Jacobson CA, Hunter BD, Redd R, et al. Axicabtagene Ciloleucel in the Non-Trial Setting: Outcomes and Correlates of Response, Resistance, and Toxicity. J Clin Oncol 2020; 38(27):3095-3106.
- Nastoupil LJ, Jain MD, Feng L, et al. Standard-of-Care Axicabtagene Ciloleucel for Relapsed or Refractory Large B-Cell Lymphoma: Results From the US Lymphoma CAR T Consortium. J Clin Oncol 2020; 38(27):3119-3128.
- Levine BL, Miskin J, Wonnacott K, Keir C. Global Manufacturing of CAR T Cell Therapy. Molecular Therapy 2017; 4:92-101.
- Vormittag P, Gunn R, Ghorashian S, Veraitch FS. A guide to manufacturing CAR T cell therapies. Curr. Op. Biotechnology 2018; 53:164-181.
- Iyer RK, Bowles PA, Kim H, Dulgar-Tulloch A. Industrializing Autologous Adoptive Immunotherapies: Manufacturing Advances and Challenges. Front. Med. 2018; 5:150.
- Harrison RP, Zylberberg E, Ellison S, Levine BL. Chimeric antigen receptor–T cell therapy manufacturing: modelling the effect of offshore production on aggregate cost of goods. Cytotherapy 2019; 21(2):224-233.
- Roddie C, O'Reilly M, Pinto JDA, Vispute K, Lowdell M. Manufacturing chimeric antigen receptor T cells: issues and challenges. Cytotherapy 2019; 21(3):327-340.
- Tyagarajan S, Schmitt D, Acker C, Rutjens E. Autologous cryopreserved leukapheresis cellular material for chimeric antigen receptor–T cell manufacture. Cytotherapy 2019; 21(12):1198-1205.
- Charrot S, Hallam S. CAR-T Cells: Future Perspectives. Hemasphere 2019; 3(2):e188.
- Townsend MH, Shrestha G, Robison RA, O’Neill KL. The expansion of targetable biomarkers for CAR T cell therapy. J. Exp. & Clin. Cancer Research 2018; 37:163
- Bagley SJ, O’Rourke DM. Clinical investigation of CAR T cells for solid tumors: Lessons learned and future directions. Pharmacology & Therapeutics 2020; 205:107419
- Nietfeld JJ, Pasquini MC, Logan BR, Verter F, Horowitz MM. Lifetime Probabilities of Hematopoietic Stem Cell Transplantation in the U.S. BBMT 2008; 14(3):316-322.
- Mazzi MT, Hajdu KL, Ribeiro PR, Bonamino MH. CAR-T cells leave the comfort zone: current and future applications beyond cancer. Immunotherapy Advances 2021; 1(1):epub
- CellTrials.org Subscribe to ALL Cellular Immunotherapy Companies. Data Product Last updated 2021-02
- Bersenev A, Verter F. Where to Find Companies Developing Allogeneic CAR-Immunotherapy. CellTrials.org News Published 2020-10-24
- Gracell Biotechnologies. Gracell Biotechnologies Announces Presentation of First-in-Human Data of GC012F a First-in-Class FasTCAR-enabled Dual-targeting BCMA/CD19 CAR-T Cell Therapy for Patients with Relapsed or Refractory Multiple Myeloma at 2020 ASH Annual Meeting. PRNewswire Published 2020-12-05
- Gluckman E, Broxmeyer HE, Auerbach AD, ... Boyse EA. Hematopoietic Reconstitution in a Patient with Fanconi's Anemia by Means of Umbilical-Cord Blood from an HLA-Identical Sibling. NEJM 1989; 321:1174-1178.
- Rubinstein P, Rosenfield RE, Adamson JW, Stevens CE. Stored placental blood for unrelated bone marrow reconstitution. Blood 1993; 81(7):1679–1690.
- Cryo-Cell. Corporate Overview and Mission. Web page. Accessed 2021-04-01
- Cord Blood Registry. How One Mom Helped Launch Cord Blood In Regenerative Medicine. Blog Published 2019-11
- Min K, Song J, Kang JY, Ko J, Ryu JS, Kang MS, Jang SJ, Kim SH, Oh D, Kim MK, Kim SS & Kim M. Umbilical Cord Blood Therapy Potentiated with Erythropoietin for Children with Cerebral Palsy: A Double-blind, Randomized, Placebo Controlled Trial. Stem Cells 2013; 31(3):581-591.
- Kang M, Min K, Jang J, Kim SC, Kang MS, Jang SJ, Lee JY, Kim SH, Kim MK, An SSA, & Kim, M. Involvement of Immune Responses in the Efficacy of Cord Blood Cell Therapy for Cerebral Palsy. Stem cells and development 2015; 24(19):2259-2268.
- Sun JM, Song AW, Case LE, Mikati MA, Gustafson KE, Simmons R, Goldstein R, Petry J, McLaughlin C, Waters‐Pick B, Chen LW, Wease S, Blackwell B, Worley G, Troy J, & Kurtzberg J. Effect of Autologous Cord Blood Infusion on Motor Function and Brain Connectivity in Young Children with Cerebral Palsy: A Randomized, Placebo‐Controlled Trial. Stem Cells Translational Medicine 2017; 6(12):2071-2078
- Min K, Suh MR, Cho KH, Park W, Kang MS, Jang SJ, Kim SH, Rhie S, Choi JI, Kim HJ, Cha KY, & Kim MY. Potentiation of cord blood cell therapy with erythropoietin for children with CP: a 2×2 factorial randomized placebo-controlled trial. Stem Cell Research & Therapy 2020; 11:509
- Dawson G, Sun JM, Davlantis KS, ... Kurtzberg J. Autologous Cord Blood Infusions Are Safe and Feasible in Young Children with Autism Spectrum Disorder: Results of a Single‐Center Phase I Open‐Label Trial. Stem Cells Trans. Med. 2017; 6(5):1332-1339.
- Dawson G, Sun JM, Baker J, ... Kurtzberg J. A Phase II Randomized Clinical Trial of the Safety and Efficacy of Intravenous Umbilical Cord Blood Infusion for Treatment of Children with Autism Spectrum Disorder. J. Pediatrics 2020; 222:164-173.e5
- Kurtzberg J, Dawson G, Troy J, Sun J. Methods for the treatment of autism spectrum disorders. Patent US20200069741A1 Patent submitted 2017-03-13, Patent granted 2021-02-09.
- Centers for Disease Control and Prevention. Data and Statistics for Cerebral Palsy. CDC website Last reviewed 2020-12
- Centers for Disease Control and Prevention. Data & Statistics on Autism Spectrum Disorder. CDC website Last reviewed 2020-09
- Ikonomou L, Levine AD, Hematti P, Levine BL. Cell Banking for Cell and Gene Therapy: Regulatory, Ethical, and Scientific Considerations. 2020; BioProcess International Published 2020-01-31
- Allen ES, Stroncek DF, Ren J, et al. Autologous lymphapheresis for the production of chimeric antigen receptor T cells. Transfusion 2017; 57(5):1133-1141.
- Vairy S, Garcia JL, Teira P, Bittencourt H. CTL019 (tisagenlecleucel): CAR-T therapy for relapsed and refractory B-cell acute lymphoblastic leukemia. Drug Des. Devel. Ther. 2018; 12:3885–3898.
- Jalowiec KA, Pabst T, Bocksrucker C, ... Zeerleder SS. How to Collect the Minimum-Targeted CD3+ Cells for CAR-T Therapy- the Bern Approach. Blood 2019; 134(S1):2457.
- Gunter KC, Caplan AL, Mason C, Salzman R, Janssen WE, Nichols K, Bouzas LF, Lanza F, Levine BL, Rasko JEJ, Shimosaka A, Horwitz E. Cell therapy medical tourism: Time for action Cytotherapy 2010; 12:965–968.
- Joint statement of ISCT & co-signing organizations. Patient Advisory for Stem Cell Therapy and Medical Tourism. ISCT website. Published 2013-08
- ISCT Presidential Task Force. On Unproven Cellular Therapies 2015: Talking About Unproven Cell-Based Interventions. Cytotherapy 2016; 18:113-148.
- ISCT. ISCT issues patient advice and concern on unproven T-cell preservation services. ISCT Press Release. Published 2019-08-07
- ISCT. ISCT Statement of Concern Regarding Speculative Commercial Cell Banking Services. ISCT Press Release. Published 2019-10-21
- English D, Andersen BR. Single-step separation of red blood cells, granulocytes and mononuclear leukocytes on discontinuous density gradients of Ficoll-Hypaque. Journal of Immunological Methods 1974; 5(3):249-252.
- Kim C, Ellis J, Truong S, Perea J, Contreras Z, Miller J, Coelho P. Peripheral Blood Mononuclear Cell Isolation Protocol. Thermogenesis White Paper Publication date unknown.
- ThermoGenesis. ThermoGenesis Holdings Updates 510(K) Listing with the FDA to Include its Next Generation PXP®-1000 System for Advanced Cellular Processing. Press release Published 2019-12-10
- ISCT North America Legal & Regulatory Affairs Committee. Apheresis Product Procurement for further manufacturing : Best Practices and cGMP Considerations. Webinar Held 2021-03-31