You are here
Growing MSC in Bioreactors: Part 1. Key Parameters of the Bioprocessing
At University College London, the group under Dr. Qasim Rafiq is optimizing the large scale expansion of human mesenchymal stem/stromal cells (MSC) in bioreactors.
Clinical trials in cell and gene therapy frequently rely on the action of MSC, due to their immunomodulatory properties and multi-differentiation ability. It is not always clear if the therapeutic effect comes directly from the MSC themselves, or from other mediators of cellular communication that are associated with MSC and which are called the secretome. The secretome might include: lipids, mRNA, growth factors and cytokines, as well as exosomes and microvesicles. If the secretome is therapeutically effective, it can be used to deliver cell-free therapy.
To achieve the number of cells usually needed for clinical use, robust, automated, and cost effective expansion systems need to be developed. The engineering challenge in our laboratory is to produce MSC at a commercially-relevant scale and to minimize process variability and human handling.
Due to their adherent nature, MSC are usually expanded attached to surfaces. The routine procedure to expand MSC for clinical applications has been to grow them in stacks of monolayer culture chambers, also known as cell factories. This approach has several disadvantages. The high need for skilled labor during the expansion stage leads to high production costs. Additionally, monolayer processes rely on incubators to maintain the culture temperature and CO2 levels, and this method has limited process monitoring and control. Finally, monolayer expansion systems have limited scalability, an important concern when manufacturing cells for allogeneic cell and gene therapies.
To overcome these problems, the engineering approach is to expand the MSC in bioreactors. There are several types of bioreactors with very different properties. Again, because MSC are adherent, when they are expanded in a bioreactor they require a surface to attach to during growth. This is achieved using bioreactors with structures for cell attachment, and these structures can include hollow fiber bioreactors, fixed/packed bed bioreactors, or stirred tank bioreactors that hold microcarriers (small beads with dimensions between 100 to 250 µm).
In addition to the type of bioreactor and type of growth matrix that is used, other bioprocesses parameters that must be evaluated when establishing a manufacturing process for MSC include temperature, oxygen, medium formulation/supplements, and agitation, among others. All of these parameters have impacts on cell yield and cell potency.
Each bioreactor has its own characteristics, but all of them present several advantages over monolayer culture systems. Bioreactors reduce human handling and do not depend on other pieces of equipment to control the temperature. Medium exchange is also easier to perform in bioreactors when compared to monolayer cultures. Another advantage of some bioreactors is their scalability. When developing an allogeneic cell therapy, bioreactors can be operated in scales from 100 mL up to 5 or 50 L. Manufacturing the MSC at large scale brings down the production costs and makes the therapy more affordable.
We are preparing a series of articles to examine the key parameters to be considered when developing an MSC manufacturing process based on microcarriers and bioreactors, and their potential impact on the final product. This diagram outlines the steps of the process, and below we enumerate the key questions that arise during each step, to be revisited in the following articles of this series.
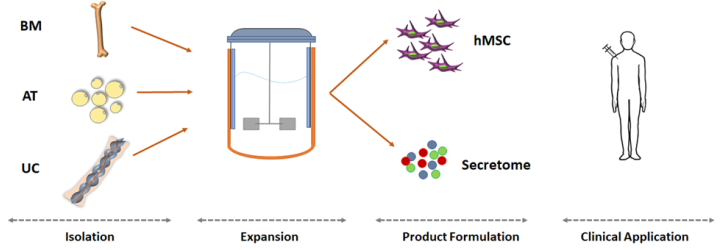
Isolation
- Optimizing isolation protocols
- What transport conditions maximize isolation success rates?
- Do different sources of MSC yield cells with different clinical potential?
- Donor-to-donor variability
Expansion
- How to optimize bioprocesses to meet the doses required?
- What is the impact of expansion bioprocesses on cell potency?
- How to optimize bioprocesses towards the production of a cell-free product?
Product Formulation
- How to define a dose for cell-free products?
- What assays can be defined for cell-free therapies?
- What type of cryoprotectant solutions need to be used for cell-free products?
Clinical Application
- What dose would optimize clinical outcomes?
- What is the most suitable administration route?
- What indicators should be used to compare the clinical outcome of novel therapies with established ones?



