You are here
The Evolution of Cord Blood Processing and the Role of PrepaCyte
The cord blood industry has been undergoing a big shift in the procedures that banks employ to process cord blood prior to cryopreservation. This makes it very timely to review what methods of cord blood processing were traditionally used in the past, how their popularity has evolved with time, what is driving the current changes, and where may the industry be headed in the future?
Early History of Cord Blood Processing
On the heels of the world’s first cord blood transplant to treat Matt Farrow in 19881, the world’s first cord blood banks were established. The very first bank was established by the New York Blood Center, and that effort was led by Dr. Pablo Rubinstein2. He invented a method of cord blood processing that was called the “Rubinstein method” and which is still widely used today3. The Rubinstein method of processing cord blood is to manually add the chemical hydroxyethyl starch to the blood and wait for red blood cells to sediment out of the blood4. This chemical is commonly known as “hetastarch”, or by the brand name “Hespan”, and is also abbreviated as HES. Following this sedimentation, the blood is spun in a centrifuge to further fractionate components. The plasma is usually discarded, while the “buffy coat” layer that contains white blood cells and stem cells is the component that goes into cryogenic storage5.
As the cord blood industry started to grow in the mid-1990s, medical devices were invented which semi-automated the processing of cord blood. The concept of these devices is that a bag holding the cord blood is placed into the device, the blood is transferred via sterile tubes to a chamber, where the device uses centrifugal action to separate blood components, then the buffy coat is drained off into a separate bag using an optical sensor to detect changes in components, and the final bag holding the buffy coat goes into storage6. The beauty of these systems is that they are mostly automated: the staff may have to transfer cord blood from the collection bag to the processing bag, but from that point onwards they never have to conduct a manual transfer of blood or cells during processing. The final processing bag is designed to be used for cryogenic storage.
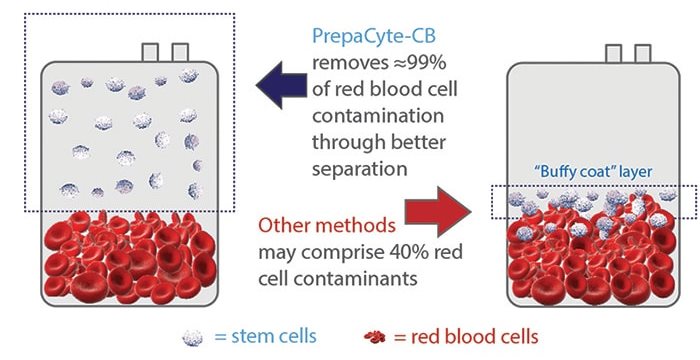
The invention of PrepaCyte to replace hetastarch was a significant innovation to the field of manual cord blood processing7. PrepaCyte®-CB is a reagent that is injected into the collection bag, then the bag is shaken to mix the contents and simply hangs for a half hour8. PrepaCyte®-CB is dramatically more efficient than hetastarch at separating red cells because the sedimentation process is different. PrepaCyte®-CB allows for the red cells to settle while keeping the white cells in the plasma suspension, instead of forming a buffy coat layer containing white cells. With the traditional processing methods that lead to the formation of a buffy coat, it is tricky to correctly separate Total Nucleated Cells (TNC) from Red Blood Cells (RBC) because there is cross contamination at the line between the buffy coat layer and the RBC layer (see the diagram)9. Without the buffy coat it is easier and more precise to express the plasma containing white cells down to the red cell line. This allows for higher recovery of stem and progenitor cells and maximum depletion of RBC. Several comparisons of cord blood processing methods that were published around 2010 showed that PrepaCyte®-CB attained an RBC depletion over 98%, while achieving recoveries of white cells and stem cells comparable to other processing methods10-12.
Engraftment Comparisons
Some studies have looked at the clinical benefits, as measured by transplant outcomes, between the processing methods used to retrieve stem cells from cord blood units. These studies have all been retrospective.
The Center for International Blood & Marrow Transplant Research (CIBMTR) compared the outcomes of transplants with cord blood units provided by the 16 public cord blood banks in the USA, for 530 patients receiving cord blood transplants for AML leukemia. They found that neutrophil recovery is significantly better when red blood cells are thoroughly removed, but their study did not find differences in survival that correlated with cord blood processing method13. A follow up study looked at outcomes versus processing for 133 patients receiving double cord blood transplants, and again found that survival was not linked to processing method14.
A more tightly focused study looked at the outcomes of 745 transplants that all came from the same cord blood bank, where the only difference in processing was between the sedimentation reagent hetastarch (brand name Hespan) versus PrepaCyte®-CB. That study found faster engraftment with PrepaCyte®-CB, but there was no significant difference in overall patient survival15,16.
Companies Providing Cord Blood Processing
A decade ago, the two main competitors in the arena of semi-automated processing were the Sepax® and AXP® devices6. In 2015, the Parent's Guide to Cord Blood Foundation surveyed family cord blood banks for an industry report and found that their breakdown of processing methods was 43% Automated, 36% Manual, and 21% No Answer17. Among the family banks using automation, 74% were using Sepax®17. In addition, most public cord blood banks accredited by FACT-Netcord were also using Sepax®18. The high adoption rate of automated processing was remarkable given that these methods were much more expensive than manual processing in terms of purchasing the device and then repeatedly purchasing disposable supplies. Each bank which was relying on a semi-automated device had to purchase two devices, in case one failed. Also, for those banks located in remote parts of the world, the disposable kits could be quite expensive and subject to lengthy shipping delays. Nonetheless, cord blood bank managers have told Parent's Guide to Cord Blood Foundation that the automated processing was very attractive if the bank had difficulty hiring and retaining staff that had the proper training for manual processing of cord blood.
Over the past decade, there has been a great deal of corporate restructuring among the companies that manufacture processing technologies for cord blood banks, and this has led to changes in which technologies are available and how they are licensed.
 The PrepaCyte®-CB technology was patented by the company BioE, which rebranded as CytoMedical Design Group, and then sold PrepaCyte®-CB to Cryo-Cell International in 201519,20. They believe that banks which use the PrepaCyte®-CB processing method have a competitive advantage. Consequently, Cryo-Cell will only license PrepaCyte®-CB to public banks or to family banks that do not compete with them.
The PrepaCyte®-CB technology was patented by the company BioE, which rebranded as CytoMedical Design Group, and then sold PrepaCyte®-CB to Cryo-Cell International in 201519,20. They believe that banks which use the PrepaCyte®-CB processing method have a competitive advantage. Consequently, Cryo-Cell will only license PrepaCyte®-CB to public banks or to family banks that do not compete with them.
The AutoXpress® Platform, or AXP® device, was developed by the company Thermogenesis. This company was founded in 1989 by Philip Coelho, who collaborated closely with Dr. Pablo Rubinstein to develop their technology21-23. But Thermogenesis has undergone multiple incarnations over the years. Originally, they were publicly traded on the Nasdaq under ticker symbol KOOL. In 2013 Thermogenesis merged with privately owned TotipotentRx to form the new company Cesca Therapeutics (Nasdaq: KOOL)24. In 2017 Cesca acquired their main competitor SynGen, also founded by Philip Coelho21,25. Then, effective Nov. 2019, Cesca changed their name to Thermogenesis Holdings and changed their Nasdaq ticker symbol to THMO26. Since Nov. 2017, the CEO of Thermogenesis has been Xiaochun (Chris) Xu, PhD, MBA27. Under Dr. Xu, Thermogenesis Holdings is linked to multiple other companies that he leads or advises, including the Chinese company BoyaLife Group which banks cord blood and placenta. The stock price of Thermogenesis Holdings fell precipitously between 2021 and 2023. During that time, Thermogenesis branched into new activities, developing devices for immune cell banking and for automated manufacturing of CAR-T cells28. Some analysts believe that Thermogenesis Holdings is poised for an economic rebound in 202429.
The Sepax® device was developed by the Swiss company Biosafe SA30. Although their patent was granted about a decade after the AXP device, we saw from the 2015 industry survey of Parent's Guide to Cord Blood Foundation that Sepax had a more effective sales team and captured a notable fraction of the market for semi-automated cord blood processing technology17. The Sepax manufacturer Biosafe SA was acquired by GE Healthcare in 201631. Then, in 2020, GE spun off its life sciences division by selling it to Danaher Corporation, which rebranded GE Healthcare as Cytiva32. But, in late 2022, Cytiva made the “business decision” to leave the medical device business. There was no press release for this; the decision was communicated by email to individual customers that had Sepax devices. Subsequently, Cytiva and Pall Life Sciences merged in 2023, and in 2024 they released a new medical device named SefiaTM for automated manufacturing of CAR-T33,34.
Recent Upheavals in Cord Blood Processing
The Cytiva decision to stop supporting the Sepax medical device has had devastating consequences for cord blood banks around the world. Basically, everyone who was processing with the Sepax semi-automated device had to switch to another method. To the best of our knowledge, the vast majority of former Sepax customers have switched to manual sedimentation with the chemical hetastarch.
The second blow to hit the cord blood industry is a worldwide shortage of the chemical hetastarch, which is not going to be alleviated anytime soon. The processing of cord blood only requires small quantities of hetastarch, whereas the primary market for this chemical was in medical applications. For many years, emergency room physicians have given large infusions of hetastarch to patients who were going into shock from blood loss, as a substitute for blood transfusions when they did not have enough time to get one. But within the past decade, follow up reviews have found that too many of those patients subsequently developed kidney failure35. Of course, medical practice guidelines were revised, and physicians in accredited hospitals were trained not to give hetastarch to patients that were “vulnerable or critically ill”36. Somehow, these additional safety measures were not enough, and a fourth practice review by the European Medicines Agency (EMA) found that physicians were still over-prescribing hetastarch. The upshot is that the EMA flat out banned hetastarch in February of 202236. In the US, the FDA still allows hetastarch, but it has a black box warning on the package insert37. B. Braun Medical, the company that manufactured hetastarch under the brand name Hespan, discontinued the product in 2022, and this has triggered a shortage which persists today38,39.
2024 Automation of PrepaCyte®-CB
During 2023 the laboratories of cord blood banks around the world were hit with a double punch: Banks that were using Sepax processing were forced to change their methods of depleting red cells and expressing plasma, but simultaneously there was a shortage of the most popular reagent for manually depleting red cells from cord blood. Not surprisingly, many laboratory managers and device developers are currently taking a serious look at alternative methods of cord blood processing. Cord blood banks have demonstrated that they are willing to invest in processing equipment that semi-automates the tasks of laboratory technicians. Therefore, Cryo-Cell International has endeavored to bring the PrepaCyte®-CB processing technology to this segment of the market by developing a semi-automated system for processing cord blood with PrepaCyte®-CB.

Cryo-Cell International has partnered with Macopharma, the manufacturer of the MacoPress Smarter automated blood separation device, to develop a semi-automated workflow for processing with PrepaCyte®-CB40-42. Combining these two technologies, the two stages of cell expression are now automated. First, after the PrepaCyte®-CB reagent causes the red cells to sediment to the bottom of the blood bag, the plasma and white cells are expressed automatically. Second, there is a centrifuge cycle to separate the plasma from the white cells, and then the plasma is automatically expressed. The replacement of manual labor with automated separation of blood components allows for a more reproducible final product. The MacoPress Smarter blood separation device, which has US FDA 510K device clearance, integrates with common blood bags and has a data management system to provide full traceability of every separation43.
At Cryo-Cell International, in-house testing of the semi-automated system that marries PrepaCyte®-CB with the MacoPress Smarter have yielded results comparable to manual PrepaCyte®-CB processing conducted by an experienced technician. These results are consistent with the RBC depletions and TNC recovery rates that have been published in third-party comparisons of processing systems6,16,44. Cryo-Cell International invites interested cord blood banks to contact them for demonstrations of this system, performance data, and licensing inquiries.
References
- Gluckman E, Broxmeyer HE, Auerbach AD, Friedman HS, Douglas GW, Devergie A, ... Boyse EA. Hematopoietic Reconstitution in a Patient with Fanconi's Anemia by Means of Umbilical-Cord Blood from an HLA-Identical Sibling. NEJM 1989; 321(17):1174-1178.
- Rubinstein P, Rosenfield RE, Adamson JW, Stevens CE. Stored placental blood for unrelated bone marrow reconstitution. Blood. 1993; 81(7):1679–1690.
- Rubinstein P, Dobrila L, Rosenfield RE, Adamson JW, Migliaccio G, Migliaccio AR, Taylor PE, Stevens CE. Processing and cryopreservation of placental/umbilical cord blood for unrelated bone marrow reconstitution. Proc Natl Acad Sci USA. 1995; 92(22):10119–10122.
- Scaradavou A. Why red blood cells should be removed before cord blood storage. Parent's Guide to Cord Blood Foundation Newsletter. Published 2014-11
- Sapkota A. Buffy Coat- Definition, Preparation, Uses. Microbe Notes. Published 2023-08-03
- Solves P, Planelles D, Mirabet V, Blanquer A, Carbonell-Uberos F. Qualitative and quantitative cell recovery in umbilical cord blood processed by two automated devices in routine cord blood banking: a comparative study. Blood Transfusion. 2013; 11(3):405–411.
- BioE. BioE Awarded Patent For Cell Separation Compositions Used To Obtain Company's Category Defining Cord Blood Stem Cell. BioProcess Online News. Published 2007-01-16
- Cryo-Cell International. PrepaCyte®-CB Cord Blood Processing System Procedure Summary. Web Instructions for Use (not product insert). Accessed 2024-06-01
- Cryo-Cell International. Why the processing method for cord blood matters. Blog. Accessed 2024-06-01
- Hudspeth D. Evaluation of PrepaCyte®-CB as an alternative to hetastarch methods for processing umbilical cord blood samples. ISCT Telegraft. 2008; 15(4):4-6.
- Basford C, Forraz N, Habibollah S, Hanger K, & McGuckin C. The cord blood separation league table: a comparison of the major clinical grade harvesting techniques for cord blood stem cells. Intnl J Stem Cells. 2010; 3(1):32–45.
- Regan D, Wofford J, Fortune K, Henderson C, Akel S. Clinical Evaluation of an Alternative Cord Blood Processing Method. Poster presented at AABB Annual Meeting 2011.
- Ballen KK, Logan BR, Laughlin MJ, He W, Ambruso DR, Armitage SE, ... Eapen M. Effect of Cord Blood Processing on Transplantation Outcomes after Single Myeloablative Umbilical Cord Blood Transplantation. Biol. Blood Marrow Transpl. 2015; 21(4):688-695.
- Nikiforow S, Li S, Snow K, Liney D, Kao GSH, Haspel R, ... Ballen KK. Lack of impact of umbilical cord blood unit processing techniques on clinical outcomes in adult double cord blood transplant recipients. Cytotherapy 2017; 19(2):272-284.
- Nadimpalli S, Buchanan P, Bloomquist J, Ferguson W, Regan D, Freter C, Babic B, Lionberger J. Comparison of Processing Reagents (Hespan and PrepaCyte-CB®) in Preparation of Cord Blood Units at the Saint Louis Cord Blood Bank. Biol. Blood Marrow Transpl. 2017; 23(S3):S174-S175.
- Babic A, Buchanan P, Gill A, Bloomquist J, Regan D, Bhatla D, Ferguson W. Analysis of outcomes of single-unit cord blood transplantation with umbilical cord blood units processed with two different red blood cell sedimentation reagents. Transfusion. 2021; 61:1856-1866.
- Verter F, Silva Couto P. Cord Blood Industry Report. Parent's Guide to Cord Blood Foundation Newsletter Published 2015-11
- Sepax system - the essential choice. Sepax technology brochure. Published 2017
- Bioprocess Online. BioE Awarded Patent For Cell Separation Compositions Used To Obtain Company's Category Defining Cord Blood Stem Cell. News. Published 2007-01-16
- MarketScreener. Cryo-Cell International, Inc. completed the acquisition of specified assets of Prepacyte®-CB cord blood business from CytoMedical Design Group LLC for $2.9 million. Press. Published 2015-06-29
- Philip Coelho. Linked IN profile. Accessed 2024-06-01
- Dobrila L, Jiang S, Chapman J, Marr D, Kryston K, Rubinstein P. Automated separation of cord blood MNC fraction in a closed system: Thermogenesis AXP™ system. Transplant Cellular Therapy (ASTCT journal). 2006; 12(S2):104.
- BioSpace. ThermoGenesis Granted Three Additional Patents by the U.S. Patent & Trademark Office. News. Published 2007-03-12
- Levin J. ThermoGenesis and TotipotentRx Announce Definitive Merger Agreement. Fierce Biotech. Published 2013-07-16
- BioSpace. Cesca Therapeutics Acquires The Cell Processing Systems Of SynGen Inc. Under Asset Acquisition Agreement. News. Published 2017-07-10
- Cesca Therapeutics. Cesca Therapeutics To Change Name And Ticker Symbol To Reflect New Strategic Focus. PRNewswire. Published 2019-10-31
- Simply Wall St. ThermoGenesis Holdings Management. Commentary. Published 2024-02-29
- Hargreaves B. ThermoGenesis launches automated cell therapy CDMO. BioPharma Reporter. Published 2022-03-31
- Simply Wall St. Analysts Expect ThermoGenesis Holdings, Inc. (NASDAQ:THMO) To Breakeven Soon. Commentary. Published 2024-02-29
- Google Patents. Sequential processing of biological fluids. Patent.
- GE Healthcare. Investing in Cures for Cancer: GE Poised to Lead Industrialization of Cell Therapy Industry with Acquisition of Biosafe Group SA. BusinessWire. Published 2016-07-13
- Hargreaves B. GE Healthcare Life Sciences completes transition into Cytiva. BioPharma Reporter. Published 2020-04-01
- Cytiva. Cytiva and Pall Life Sciences complete integration to create a global innovation and solutions leader in biotechnology. News. Published 2023-05-02
- Cytiva. Deliver what’s next in autologous CAR T manufacturing. Web page. Accessed 2024-06-01
- Zarychanski R, Abou-Setta AM, Turgeon AF, Houston BL, McIntyre L, Marshall JC, Fergusson DA. Association of hydroxyethyl starch administration with mortality and acute kidney injury in critically ill patients requiring volume resuscitation: a systematic review and meta-analysis. JAMA 2013; 309(7):678-688.
- European Medicines Agency (EMA). PRAC recommends suspending hydroxyethyl-starch solutions for infusion from the market. News. Published 2022-02-11
- US Food and Drug Administration (FDA). Hespan package insert. Accessed 2024-06-01
- US Federal Register. B. Braun Medical, Inc.; Withdrawal of Approval of Abbreviated New Drug Application of Hydroxyethyl Starch. Notice. Effective 2022-10-22
- American Society Health-System Pharmacists (ASHP). Current Drug Shortages. Hydroxyethyl Starch. Last updated 2024-05-02
- Johnson P. Evaluation of the MacoPress SMART Blood Component Separator for Volume Reduction of Cord Blood Units in a Multicenter Validation Study. Stem Cells Transl. Med. 2018; 7(S1):S17.
- Maheux A, Trépanier P, Fournier D, Cloutier M, Ducas E, Boyer L, Jacques A. Validation of the MacoPress SMART for Volume Reduction of Cord Blood Units at Héma‐Québec's Cord Blood Bank. Stem Cells Transl Med. 2019; 8(S1):S30.
- MacoPress Smarter. Accessed 2024-06-01
- US Food and Drug Administration (FDA). BK180306 - MacoPress Smart Smarter and DMS Plus. Substantially Equivalent 510(k) Device Information. Last updated 2019-01-31
- Henderson C, Wofford J, Fortune K, Regan D. Evaluation of Processing Technologies for Umbilical Cord Blood (UCB). Poster presented at ISCT North America Meeting 2010.


