You are here
Current uses of Cord Blood for Immunotherapy
There is optimism in the cord blood community that cord blood units can be used as source material to develop cellular immunotherapies, creating a new application for cord blood. Immunotherapy is a relatively new field of medicine in which the native cells of the immune system are multiplied or modified so that they do a better job of fighting cancer or viruses. It has become customary for every cord blood conference to include a speaker who is working with T-cells sourced from cord blood, and a speaker using NK cells sourced from cord blood.
But just how common is immunotherapy with cells sourced from cord blood? The purpose of this article is to give an overview of the field, with the caution that the field is rapidly evolving.
As Dr. Kurtzberg is fond of saying, a cord blood unit is “not just a bag of stem cells.” Specifically, cord blood contains more than hematopoietic (blood-forming) stem cells. There are a variety of other blood and immune system cells that are present in or can be derived from a cord blood unit, including:
- regulatory T cells (T-regs),
- virus-specific T-cells (VST),
- cytoxic T lymphocytes (CTL),
- chimeric antigen receptor modified T-cells (CAR-T), plus
- natural killer cells (NK) and CAR-NK cells.
Over the past decade, several companies have developed expanded cord blood products that can engraft faster during a stem cell transplant. A handful of these companies have received expedited approval tracks, and we recently featured a story about the completed phase 3 trial of the product Omidubicel from the company Gamida Cell.
It is also possible to culture mesenchymal stromal cells (MSC) from cord blood, and some companies have product pipelines based on this approach, most notably the product Cartistem® from Medipost that was approved in South Korea for knee osteoarthritis.
At the Cord Blood Connect conference in Sept. 2019, Dr. Krishna Komanduri presented a provocative representation of a cord blood unit as a hamburger with toppings: the unmanipulated cord blood is the burger itself, and the toppings are the numerous additional products that can potentially be derived from a cord blood unit (not all from the same unit).
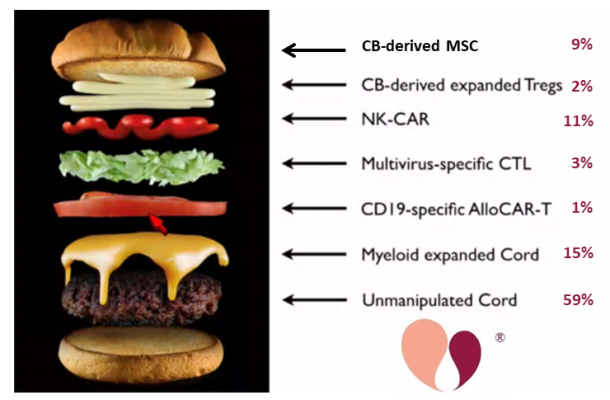
For this year’s Cord Blood Connect conference in Sept. 2020, Dr. Verter of the Parent’s Guide to Cord Blood Foundation repeated that image, assigning to each piece of the burger a percentage of the currently recruiting cord blood trials which are using that approach. The data was sourced from the Foundation’s portal of currently recruiting cord blood trials, which contained 123 cord blood trials as of 30 April 2020.
This is not visible in the image, but among the 59% of trials that employ unmanipulated cord blood, the contributions are 37% performing traditional cord blood transplants and 22% are using cord blood for regenerative medicine.
Another way to look at immunotherapy trials sourced from cord blood, is not as a fraction of cord blood trials, but as a fraction of immunotherapy trials. Currently, CAR immunotherapy is the most popular form of cell therapy and the number of CAR trials (with any cell type, either CAR-T or CAR-NK) has grown 25-fold over the past decade. The earliest CAR-T trials harvested cells from the patient and modified them in the laboratory over the course of weeks, a process that is both risky and very expensive. The latest innovation in the CAR-immunotherapy field is the development of allogeneic therapy that is available off the shelf from universal donors. In their data on allogeneic CAR-immunotherapy, the team at CellTrials.org found that 7% of 2019 CAR-immunotherapy clinical trials explicitly use a universal donor cell line, and as of March 2020, 20% of CAR-immunotherapy companies are developing universal donor products.
Right now, our portal of recruiting cord blood trials only holds a single clinical trial that employs CAR-T cells sourced from cord blood. This represents 1% of recruiting cord blood trials as of April 2020 but only about 0.1% of the recruiting CAR-immunotherapy trials as of June 2020.
While at present cells derived from cord blood are only a small contribution to the field of immunotherapy, we believe that this clinical application is on the verge of rapid growth. There are several companies that are developing cellular immunotherapy products sourced from cord blood. Some examples are Cellenkos in the United States, Glycostem in the Netherlands, and Takeda in Japan. Several banks have told Parent’s Guide to Cord Blood Foundation that they are in pre-clinical tests of CAR-T or NK cell lines sourced form cord blood. Some immunotherapy products from perinatal sources are in cell therapy trials for COVID-19. Hopefully, all of these research and development efforts will lead to an increase in the percentage of universal donor cellular immunotherapies that are sourced from cord blood.


