You are here
COVID-19 Lessons Learned for Biobanks
Summary of COVID-19 Lessons Learned for Biobanks:
|
This post is based on a June 2023 publication from a collaboration led by the teams of CellTrials.org, Dr. Guido Moll et al. from Charité University Medicine Berlin in Germany, and Prof. Otávio Cabral-Marques et al. from the University of São Paulo in Brazil. The paper compiled clinical trials of advanced cell therapy for COVID-19, traced them to the published trial outcomes, and performed a meta-analysis of the efficacy of treating COVID-19 with infusions of Mesenchymal Stromal Cells (MSC)1. This was the largest meta-analysis to date of MSC therapy for COVID-19, and builds upon previous research into the clinical guidelines for intravenous delivery of MSC products2-4. We found that MSCs provide a relative risk reduction for all-cause COVID-19 mortality of RR=0.63 [95% CI 0.46 to 0.85]1.
In this post, we summarize a different aspect of the paper: The demographics of the clinical trials and their implications for the biobanking industry. During the Coronavirus pandemic, biobanks played a crucial role as local suppliers of cell therapy products at a time when international shipping routes were disrupted.
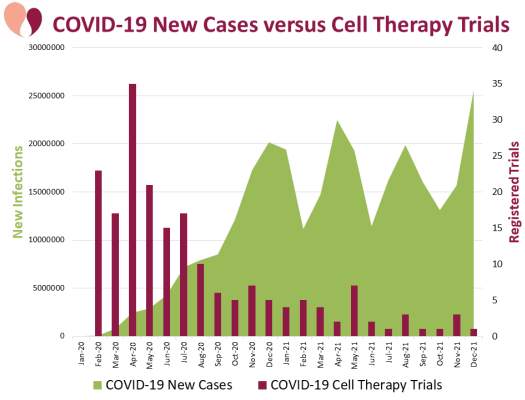 Over the two-year period from Jan. 2020 to Dec. 2021, we searched 18 international registries and found 195 clinical trials registered of cell-based therapy for COVID-19. The number of trials registered per month within this timeframe reveals that there was one strong peak of trials in April 2020, which subsided by the fall of 2020 into a long tail of a few trials per month. During the two-year time frame of 2020 and 2021, there were four worldwide surges of infections with variants of COVID-19. The large surges in infection began near the end of 2020, by which time cell therapy trials were already months past their peak. Our team is the only one that has graphed the timeline of COVID-19 cell therapy trials in this detail for this long of a time period. Our data demonstrates that trial registrations were not driven by disease prevalence. Trial registrations were more likely based on whether the lead investigators had access to a cell therapy product with an already established safety profile, which could be re-purposed as a potential therapy for COVID-19. We have continued monitoring trials beyond the timeframe of the published paper and can report that the tail of a few trials per month continues through 2022 and into early 2023.
Over the two-year period from Jan. 2020 to Dec. 2021, we searched 18 international registries and found 195 clinical trials registered of cell-based therapy for COVID-19. The number of trials registered per month within this timeframe reveals that there was one strong peak of trials in April 2020, which subsided by the fall of 2020 into a long tail of a few trials per month. During the two-year time frame of 2020 and 2021, there were four worldwide surges of infections with variants of COVID-19. The large surges in infection began near the end of 2020, by which time cell therapy trials were already months past their peak. Our team is the only one that has graphed the timeline of COVID-19 cell therapy trials in this detail for this long of a time period. Our data demonstrates that trial registrations were not driven by disease prevalence. Trial registrations were more likely based on whether the lead investigators had access to a cell therapy product with an already established safety profile, which could be re-purposed as a potential therapy for COVID-19. We have continued monitoring trials beyond the timeframe of the published paper and can report that the tail of a few trials per month continues through 2022 and into early 2023.
Among these 195 clinical trials of advanced cell therapy for COVID-19, there were 204 types of cell products tested, because some trials employed more than one cell type. Our paper characterizes these cell therapy products by various aspects of their manufacturing and clinical delivery, but the two most notable numbers are the high fraction of allogeneic products and the high fraction of MSC products. First, the cell donor was allogeneic in 76% of the trials, autologous in 11%, and unknown in 13%. Thus, at least three quarters of the trials were using cell therapy products that were sourced from healthy donors and given to COVID-19 patients. Second, this is very similar to the fraction of trials employing MSC, which was 72%. Together, these facts illustrate that many clinicians seeking to offer advanced cell therapies to their COVID-19 patients relied on access to a biobank which manufactured allogeneic MSC or allogeneic immunotherapies. Also critical to patient treatment were reliance on cryogenic cell storage and reliance on a supply chain that could deliver cell therapies that were either previously frozen or freshly manufactured5.
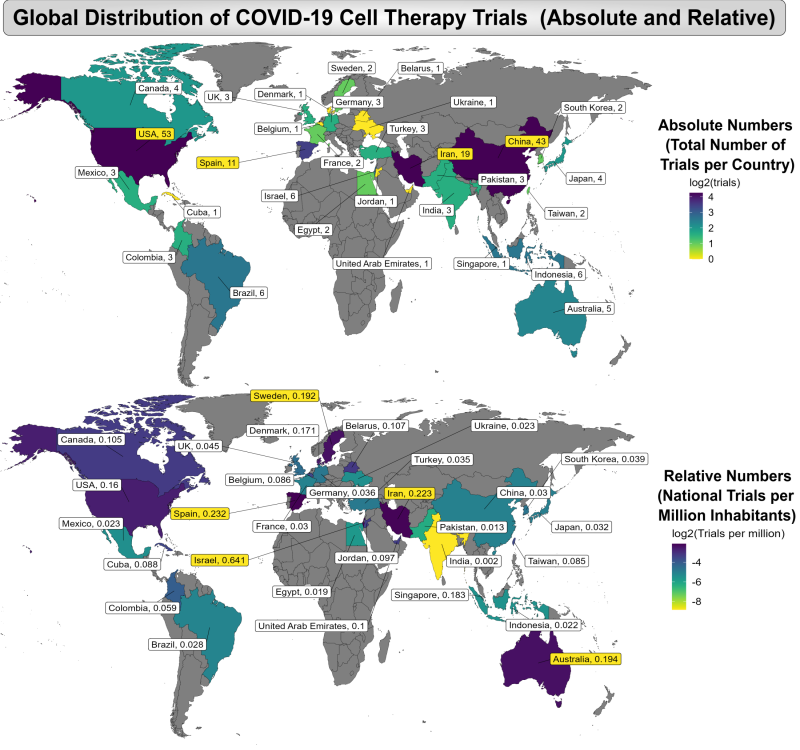
We discovered that the COVID-19 pandemic had a democratizing impact on cell therapy endeavors. We have prepared global heatmaps of the countries where clinical trials of advanced cell therapy for COVID-19 were conducted, regardless of where they were registered. Only one trial took place in more than one country. The top map displays the number of trials per country, while the bottom map displays the number of trials per million inhabitants of the country. The first amazing fact about the maps is that over this two-year period, 30 countries registered clinical trials of advanced cell therapy for COVID-19. The leaders in numbers of trials were the United States (53), China (43), Iran (19), and Spain (11), but many countries that are not typically considered to be centers of cell therapy research also participated. More insight is gained from the second map of per capita trial registrations, where the leaders are Israel (0.641), Spain (0.232), Iran (0.223), Australia (0.194), and Sweden (0.192). Our study has been the only compilation of COVID-19 cell therapy trials to thoroughly search the Iranian trial registry and report the strong participation of Iran in either numbers of trials or per capita relative numbers of trials of cell therapy for COVID-19.
We believe that the biggest lesson learned from the demographics of advanced cell therapy for COVID-19 is the critical importance of local biobanks that could rapidly deliver allogeneic cell-based therapies to seriously ill patients. Clinicians that desired to treat their COVID-19 patients with cell therapy not only needed sources of previously tested cellular products, but they also needed sources they could access during a time when many borders were closed. Professionals from various medical specialties have discussed the importance of learning lessons from COVID-19 so that we can do better during the next pandemic. In the field of cell therapy an important lesson to learn is that each country should nurture a local biobanking industry. As more cell therapy products achieve regulatory approvals, distribution networks should be established with multiple storage hubs, so that if a pandemic breaks out and closes borders, it will not completely prevent products that are manufactured in a central laboratory from reaching patients.
References:
- Pedro Silva Couto, Al-Arawe N, Filgueiras IS, Fonseca DLM, Hinterseher I, Catar RA, Chinnadurai R, Bersenev A, Cabral-Marques O, Guido Moll & Frances Verter (June 2023) Systematic Review and Meta-Analysis of Cell Therapy for COVID-19: Global Clinical Trial Landscape, Published Safety / Efficacy Outcomes, Cell Product Manufacturing and Clinical Delivery. Frontiers Immunology 14:1200180.
- Moll G, et al. Intravascular Mesenchymal Stromal/Stem Cell Therapy Product Diversification: Time for New Clinical Guidelines. Trends Mol Med 2019; 25(2):149-163.
- Moll G, et al. MSC Therapies for COVID-19: Importance of Patient Coagulopathy, Thromboprophylaxis, Cell Product Quality and Mode of Delivery for Treatment Safety and Efficacy. Frontiers Immunology 2020; 11:1091.
- Moll G, Ankrum JA, Olson SD, & Nolta JA. Improved MSC Minimal Criteria to Maximize Patient Safety: A Call to Embrace Tissue Factor and Hemocompatibility Assessment of MSC Products. Stem Cells Translational Medicine 2022; 11:2-13.
- Cottle C, Porter AP, Lipat A, et al. Impact of Cryopreservation and Freeze-Thawing on Therapeutic Properties of Mesenchymal Stromal/Stem Cells and Other Common Cellular Therapeutics. Curr Stem Cell Rep 2022; 8:72–92.


