You are here
Cerebral Palsy Response to Cell Therapy as a Function of Time
For the first time ever, the response of children with cerebral palsy to one cell therapy versus another has been compared over time, while holding other parameters of the comparison fixed1. This comparison found that initially improvement was more rapid with cord blood mononuclear cells (MNC), but by the end of a year there was no significant difference between response to cord blood MNC versus mesenchymal stromal cells (MSC) from cord tissue. There were 108 patients total, with 36 on each of three treatment arms, hence this result needs to be repeated by other researchers with larger patient samples.
One of the biggest problems that bedevils the treatment of cerebral palsy with cell therapy is that there are many variables in these studies, so that typically no two studies are the same and their results cannot be directly compared. Some of the variables involved are: the type of cerebral palsy the patients have, severity of their cerebral palsy, patient age range, choice of cell type, cell culture method for MSC, cell dose, cell delivery route, measurement system for scoring motor function improvements, blinding of the control group, and the time points at which improvements are assessed.
We already know that umbilical cord blood therapy has been proven effective as a treatment for children with cerebral palsy. Patients that received cord blood mononuclear cells (MNC) have significantly better improvements in gross motor skills than children in control groups. This was established by an Individual Participant Data Meta-Analysis (IPDMA) that was published as a conference abstract in 2023, and will soon appear as a full paper in a medical journal2. A similar meta-analysis is ongoing for cerebral palsy patients treated with MSC3.
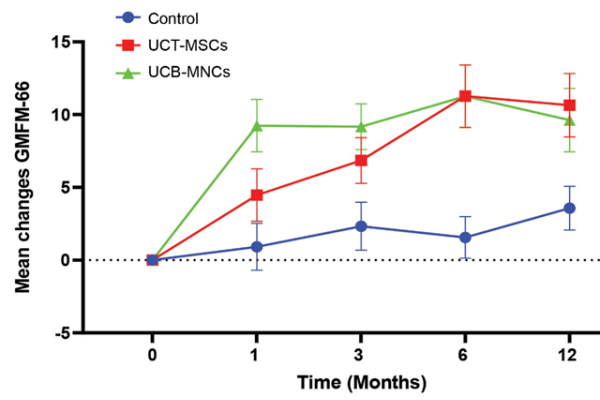
The comparison that was recently published is unique because the cord blood MNC patients and the cord tissue MSC patients were treated at the same research center and the variables were kept fixed as much as possible. All children were treated in the pediatric center of Tehran University Medical Sciences in Iran. The study participants were diagnosed with spastic cerebral palsy with white matter lesions in the brain, had severity index between 2 and 5, and had ages between 4 and 14. All children received a single intrathecal injection of either 5 million cord blood MNC per kg or a fixed dose of 20 million cord tissue MSC, or a sham procedure. The cord blood was allogeneic with a 6/6 HLA match, while the cord tissue was processed by enzymatic digestion and the MSC were cultured to the fourth passage. Primary outcomes were measured with the Gross Motor Function Measure-66 (GMFM-66), plus other metrics of function as well as various types of brain imaging, including diffusion tensor imaging. All participants, parents, and investigators were blinded during the study. Measurements were taken at one, three, six, and twelve months after the cell therapy. All participants received the same physical therapy rehabilitation program over the course of the study. Two previous publications examined the outcomes for the cord tissue MSC group and the cord blood MNC group4,5. The latest paper compares these groups versus time1.
It must be stressed that these results are preliminary, due to the small sample sizes, but the difference at one month is clearly statistically significant (P<0.05). Parents that are seeking cell therapy for their children are often concerned over which cell type will give a better response. This study suggests that in the long run cord, both blood MNC and cord tissue MSC are effective, and their outcomes are similar for children with spastic cerebral palsy. A caveat to that last statement is that the outcomes are similar when the children receive the doses and the delivery method used here. It is already known that motor skill improvements from cell therapy eventually plateau for cerebral palsy patients, so that repeated doses would be optimal to advance patient progress6,7. It is also important to point out that these two studies used intrathecal injections into the spinal cord, whereas many other hospitals and clinics rely on intravenous infusions where cells are filtered from the bloodstream by the lungs, and fewer cells cross the blood brain barrier to reach brain lesions8. This paper is an important contribution to our knowledge about cell therapy for cerebral palsy.
References
- Nouri M, Zarrabi M, Masoumi S, Khodadoust E, Majmaa A, Amanat M, Ashrafi MR, Vosough M. Cell-Based Therapy for Cerebral Palsy: A Puzzle in Progress. Cell Journal 2025; 26(9):569-574.
- Finch-Edmondson M, Paton M, Webb A, Ashrafi M, Blatch-Williams R, Cox J, Charles, et al. Umbilical Cord Blood Treatment to Improve Gross Motor Function in Individuals with Cerebral Palsy: Results from an Individual Participant Data Meta-Analysis. Stem Cells Translational Medicine. 2023; 12(Supplement_1):S6-S.
- Paton MCB, Griffin AR, Webb A, Blatch-Williams R, Novak I, Finch-Edmondson M. Exploring the Evidence Base of Mesenchymal Stromal Cells for Cerebral Palsy: A Scoping Review. Work in Progress.
- Amanat M, Majmaa A, Zarrabi M, Nouri M, Akbari MG, Moaiedi AR, ... Ashrafi MR. Clinical and imaging outcomes after intrathecal injection of umbilical cord tissue mesenchymal stem cells in cerebral palsy: a randomized double-blind sham-controlled clinical trial. Stem Cell Research & Therapy. 2021; 12:439
- Zarrabi M, Akbari MG, Amanat M, Majmaa A, Moaiedi AR, Montazerlotfelahi H, Nouri M, ... Ashrafi MR. The safety and efficacy of umbilical cord blood mononuclear cells in individuals with spastic cerebral palsy: a randomized double-blind sham-controlled clinical trial. BMC Neurology. 2022; 22(1):123
- McDonald C. Multiple Doses of Cord Blood are Better for Cerebral Palsy in Animal Model. Parent's Guide to Cord Blood Foundation Newsletter Published 2019-06
- Clowry G. Stem cell therapy for cerebral palsy: Proceeding with caution. Dev Med Child Neurol. 2022; 64(12):1434-1435.
- Rodríguez-Frutos B, Otero-Ortega L, Gutiérrez-Fernández M, Fuentes B, Ramos-Cejudo J, Díez-Tejedor E. Stem Cell Therapy and Administration Routes After Stroke. Translational Stroke Research. 2016; 7:378-387.



