You are here
Cerebral Palsy Alliance Xcellerate Program
 Cerebral palsy (CP) is a physical disability that affects movement and posture. It is a permanent life-long condition, where the brain injury is stable, but co-occurring orthopedic conditions can worsen over time. It is due to damage to the developing brain either during pregnancy or shortly after birth. Cerebral palsy is the most common physical disability in childhood. Worldwide, the incidence of cerebral palsy in high income countries is 1 in 500 births. There are currently 17 million people in the world who have cerebral palsy.
Cerebral palsy (CP) is a physical disability that affects movement and posture. It is a permanent life-long condition, where the brain injury is stable, but co-occurring orthopedic conditions can worsen over time. It is due to damage to the developing brain either during pregnancy or shortly after birth. Cerebral palsy is the most common physical disability in childhood. Worldwide, the incidence of cerebral palsy in high income countries is 1 in 500 births. There are currently 17 million people in the world who have cerebral palsy.
Cerebral Palsy Alliance (aka CP Alliance) is a nonprofit organization that provides family-centered therapies, life skills programs, equipment and support for people living with cerebral palsy and their families.
The CP Alliance is the world’s oldest and largest organization supporting families of patients with cerebral palsy. Founded by parents in Australia in 1945, the current CP Alliance helps families in Australia to navigate the National Disability Insurance Scheme (NDIS), a government-funded program that provides support services to people of all ages with disabilities. In Australia there are approximately 34,000 people with cerebral palsy, and every 15hrs an Australian child is born with cerebral palsy.
 Today, the CP Alliance Research Foundation is the leading international organization supporting research to find cures for cerebral palsy. There is no one single cause of cerebral palsy – which means there could be many ways to diagnose, treat, prevent or cure cerebral palsy. The CP Alliance Research Foundation provides funding for a team of researchers from many disciplines whose expertise, experience, and dedication are seeking answers as quickly as possible.
Today, the CP Alliance Research Foundation is the leading international organization supporting research to find cures for cerebral palsy. There is no one single cause of cerebral palsy – which means there could be many ways to diagnose, treat, prevent or cure cerebral palsy. The CP Alliance Research Foundation provides funding for a team of researchers from many disciplines whose expertise, experience, and dedication are seeking answers as quickly as possible.
The research priorities funded by CP Alliance were defined in a 2007 Delphi Study that interviewed an expert panel of consumers, researchers, and clinicians. Since 2006, the CP Alliance Research Foundation has awarded more than USD 20 million to support 179 cerebral palsy research projects in Australia and internationally. Stem cell research has been ranked by people with cerebral palsy (and their parents) as their second highest research priority after prevention of cerebral palsy. The CP Alliance both funds stem cell research on cerebral palsy and provides guidance to families considering stem cell interventions.
 Parent's Guide to Cord Blood Foundation reported in their March 2016 newsletter that CP Alliance is partnering with Cell Care, the largest family cord blood bank in Australia, on a clinical trial that offers sibling cord blood therapy at the Murdoch Childrens Research Institute to cerebral palsy patients in Australia.
Parent's Guide to Cord Blood Foundation reported in their March 2016 newsletter that CP Alliance is partnering with Cell Care, the largest family cord blood bank in Australia, on a clinical trial that offers sibling cord blood therapy at the Murdoch Childrens Research Institute to cerebral palsy patients in Australia.
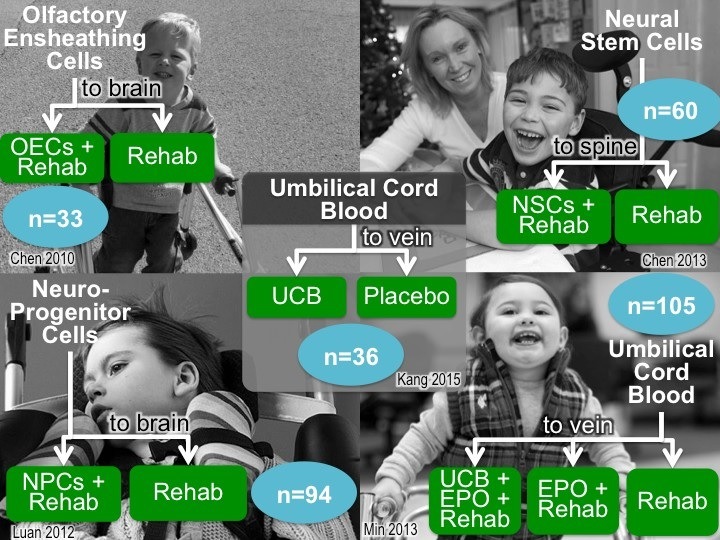 To date, five clinical trials have been published1-5 that demonstrated the effectiveness of stem cell treatment for cerebral palsy. These five studies had a cumulative enrollment of 328 patients. A meta-analysis6 led by CP Alliance Research Foundation compared these five previous studies and concluded that stem cell interventions for people with cerebral palsy had a small but statistically significant impact on gross motor skills, over the follow-up period of six months, with the most efficacy seen from umbilical cord blood stem cells. The data were insufficient and too heterogeneous to compare cognitive effects. Serious adverse events reported in these trials were low (3% stem cells; 2% controls), suggesting an acceptable benefit to risk ratio. The meta-analysis concluded that more randomized controlled trials are needed to determine which stem cell therapies have optimum impact for cerebral palsy patients.
To date, five clinical trials have been published1-5 that demonstrated the effectiveness of stem cell treatment for cerebral palsy. These five studies had a cumulative enrollment of 328 patients. A meta-analysis6 led by CP Alliance Research Foundation compared these five previous studies and concluded that stem cell interventions for people with cerebral palsy had a small but statistically significant impact on gross motor skills, over the follow-up period of six months, with the most efficacy seen from umbilical cord blood stem cells. The data were insufficient and too heterogeneous to compare cognitive effects. Serious adverse events reported in these trials were low (3% stem cells; 2% controls), suggesting an acceptable benefit to risk ratio. The meta-analysis concluded that more randomized controlled trials are needed to determine which stem cell therapies have optimum impact for cerebral palsy patients.
Below is a YouTube video of Prof Iona Novak, head of research strategies for CP Alliance, giving a talk about the Xcellerate research program. Dr. Novak points out in the video that autologous cord blood therapy for cerebral palsy is known to be safe, but a child can only use their own cord blood once. In order to enable children with cerebral palsy to receive multiple stem cell therapies, cord blood clinical trials are exploring therapy with sibling units and eventually unrelated allogeneic cord blood. In theory, children with cerebral palsy would benefit the most from neural stem cells to repair neurologic damage in the brain, but these cells do not migrate like cord blood cells and therefore must be implanted directly into the brain, which raises additional safety concerns for their administration.
The current standards of care for cerebral palsy are rehabilitation, pharmacology and orthopedic surgery. Further and longer duration trials are needed to determine if stem cell interventions should be included within the cocktail of interventions that comprise standard care. The CP Alliance believes that more stem cell research for cerebral palsy is worthwhile.
Publications
- Trial with olfactory ensheathing cells: Chen, L., Huang, H., Xi, H., Xie, Z., Liu, R., Jiang, Z., ... & Wang, H. (2010). Intracranial transplant of olfactory ensheathing cells in children and adolescents with cerebral palsy: a randomized controlled clinical trial. Cell transplantation, 19(2), 185-191.
- Trial with neuro-progenitor cells: Luan, Z., Liu, W., Qu, S., Du, K., He, S., Wang, Z., ... & Gong, X. (2012). Effects of neural progenitor cell transplantation in children with severe cerebral palsy. Cell transplantation, 21(Supplement 1), S91-S98.
- Trial with neural stem cells: Chen, G., Wang, Y., Xu, Z., Fang, F., Xu, R., Wang, Y., ... & Liu, H. (2013). Neural stem cell-like cells derived from autologous bone mesenchymal stem cells for the treatment of patients with cerebral palsy. J Transl Med, 11(1), 21.
- Trial with umbilical cord blood: Min, K., Song, J., Kang, J. Y., Ko, J., Ryu, J. S., Kang, M. S., ... & Kim, S. S. (2013). Umbilical Cord Blood Therapy Potentiated with Erythropoietin for Children with Cerebral Palsy: A Double-blind, Randomized, Placebo Controlled Trial. Stem Cells, 31(3), 581-591.
- Trial with umbilical cord blood: Kang, M., Min, K., Jang, J., Kim, S. C., Kang, M. S., Jang, S. J., ... & Kim, M. (2015). Involvement of Immune Responses in the Efficacy of Cord Blood Cell Therapy for Cerebral Palsy. Stem cells and development, 24(19), 2259-2268.
- Meta-Analysis: Novak I, Walker K, Hunt RW, Wallace E, Fahey M, Badawi N. (2016). Stem cell interventions for people with cerebral palsy: Systematic review with meta-analysis. Stem Cells Translational Medicine, 5(8) 1014–1025.



 Professor Iona Novak is the Head of Research for the Cerebral Palsy Alliance Research Institute, based at the Brain and Mind Centre, The University of Sydney. She is a Fulbright Scholar and winner of the University of Sydney Award for Professional Achievement. In 2005 she co-founded the Cerebral Palsy Alliance Research Institute for the purpose of research development and dissemination, leading to prevention, cure and reduction of adverse effects for those living with cerebral palsy. Driven by an internal belief that healthcare truly has the potential to change lives, Dr. Novak has pursued projects and roles with the greatest possible impact on children and families today and tomorrow’s world, including, establishment and leadership of the Australian Cerebral Palsy Register. Dr Novak has a background in occupational therapy, with a particular interest in neuroplasticity and stem cells. Dr. Novak was awarded Citizen of the Year, by the Australia Day Council in 2015 and named as one of the top ten most influential women in New South Wales.
Professor Iona Novak is the Head of Research for the Cerebral Palsy Alliance Research Institute, based at the Brain and Mind Centre, The University of Sydney. She is a Fulbright Scholar and winner of the University of Sydney Award for Professional Achievement. In 2005 she co-founded the Cerebral Palsy Alliance Research Institute for the purpose of research development and dissemination, leading to prevention, cure and reduction of adverse effects for those living with cerebral palsy. Driven by an internal belief that healthcare truly has the potential to change lives, Dr. Novak has pursued projects and roles with the greatest possible impact on children and families today and tomorrow’s world, including, establishment and leadership of the Australian Cerebral Palsy Register. Dr Novak has a background in occupational therapy, with a particular interest in neuroplasticity and stem cells. Dr. Novak was awarded Citizen of the Year, by the Australia Day Council in 2015 and named as one of the top ten most influential women in New South Wales.