You are here
Amniotic Membrane of the Placenta - Part 3
 This is the third and final installment in our series of articles about products made from the Amniotic Membrane of the Placenta or AMP. Specifically, we are reviewing products that supposedly contain live human cells or tissues that are intended for implantation, transplantation, infusion, or transfer into a human recipient.
This is the third and final installment in our series of articles about products made from the Amniotic Membrane of the Placenta or AMP. Specifically, we are reviewing products that supposedly contain live human cells or tissues that are intended for implantation, transplantation, infusion, or transfer into a human recipient.
In the United States, Human Cells and Tissue Products, or HCT/P’s, are regulated by the FDA’s Center for Biologics Evaluation and Research (CBER). There are two main pathways of HCT/P regulation, which are labelled by their section numbers in the Federal Code of Regulations (CFR CFR 1271.10). Most AMP products are trying to qualify for the “361 exemption” which means that so long as they are safe they can be marketed without undergoing rigorous testing to prove their efficacy for treating specific conditions. By comparison, products that do not qualify for the 361 exemption are regulated on the 351 pathway as a drug, device, and/or a biological product, and require rigorous premarket approval. In the HCT/P world today, the only stem cell injectable that the FDA explicitly approves under the 361 exemption is transplants or injections of minimally manipulated bone marrow1,2.
In order to understand the regulatory compliance of AMP products on the market today, we need to itemize the criteria needed to achieve a 361 exemption, while simultaneously being wary that each of these criteria is subject to a notable level of interpretation:
1) the HCT/P(s) are minimally manipulated
2) the HCT/P is intended for homologous use only
3) the manufacture does not involve the combination of cells or tissues with another article except as further stipulated
4) either the HCT/P…
a) is not dependent on the activity of living cells for its primary function, or,
b) if it is dependent on living cells, it can only be used in autologous, or in a first or second-degree blood relative.
Today there is some turmoil in the industry as the FDA has proposed new guidance in an effort to clarify and tighten these definitions. After public hearings in September 2016, change is likely coming that we hope will ultimately benefit both patients and the industry. So this report is based on what we know today, our opinions today, and some things we hope are coming in the future.
Every week we see an ad in our local paper, and every month the ad gets bigger. A clinical group with "stem cell" in their name is promoting free educational seminars, usually directed to seniors, to learn how Regenerative Medicine can STOP their pain. It mentions a protocol that uses the healing potential of cells derived from the Amniotic and Placental tissue. When you attend this seminar, as we did, you might be told that the cure is a miniscule .25cc injection, purported to contain "pluripotent stem cells of the amniotic membrane", and costing $5000. To our chagrin, the audience appeared eager to try it.
Many of the miraculous stem cell cures that we’re hearing so much about are indeed often attributed to the performance of living stem cells transplanted by injection. Within the U.S., living cells from unrelated humans should be regulated as drugs. However we see rapid growth in AMP-based injectable products being offered in the U.S., and targeted specifically for allogeneic use. We can only assume that those responsible for these injectable products believe they are flying safely under a 361 banner. But how can that be?
Following this experience with clinic marketing of AMP products, we contacted the Interventional Orthopedic Foundation (IOF), to learn more about the content of these products. The IOF, under the leadership of their board chair Dr. Chris Centeno, works to educate their member physicians about the safety and efficacy of stem cell products offered for orthopedic applications. The IOF conducted an investigation3,4,5,6 of several AMP products, including the one from the aforementioned seminar, and found that they contained no living stem cells. Further, the manufacturer of the product in question confirmed that it was NOT a stem cell product. Unfortunately, it was endorsed in a completely different and erroneous manner by the presenting clinic’s seminar.
Marketing of many AMP products in the U.S. is often based on unrealistic claims. To be fair, there are a variety of beneficial therapeutic components in AMP as we reported earlier: hyaluronic acid, growth factors, cytokines, proteins, and more…..and then there’s the living cells. Clinics are touting these products as a cure-all because they allegedly contain living stem cells. Yet the IOF investigation of their products found no living cells. If they did contain living cells the FDA would regulate them as drugs. These companies are operating under the FDA’s 361 exemption precisely because their AMP products have been dehydrated, sterilized… and all the cells are dead.
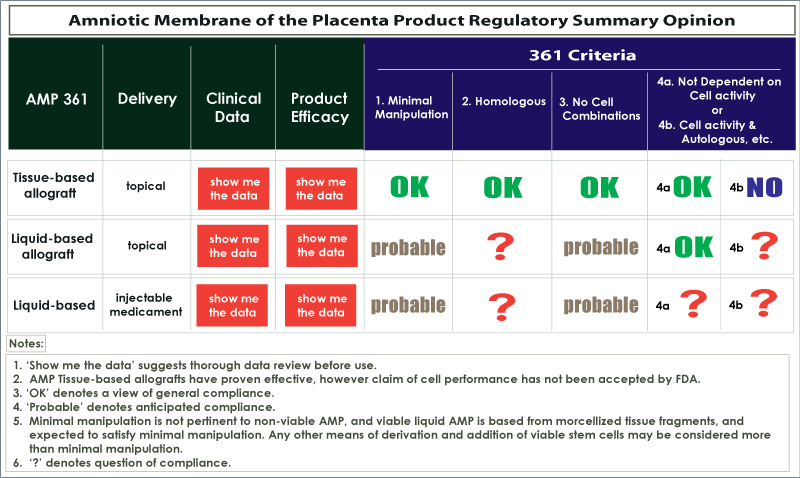
Does the absence of living cells guarantee that an allogeneic human AMP product can be marketed under the FDA 361 exemption? Products that do not contain living cells do not have to comply with HCT/P product requirements 4a and 4b listed above. As we move further into the other 361 requirements, we see an issue for the liquid group being in compliance with the definition of homologous, based on the dramatic physical transformation of the tissue into very small particles. Also the method of delivery should pass safety testing, which is a much steeper hurdle for an injectable than a topical AMP product.
Even when an AMP product does satisfy the 361 exemption, it cannot be marketed as containing living cells, because that is a false claim. We do believe that the tissue-based AMP products that are delivered topically as allografts satisfy the noted 361 criteria, 1, 2, 3 and 4a……but not 4b. The first FDA notice of potential non-compliance, called the ‘Untitled Letter’, has been sent to several AMP manufacturers that have tried to promote living cell benefit in tissue allograft products.
Let’s look again at the diagram above presenting a matrix of products and regulatory requirements. From a patient and clinician perspective, all products demand review of their clinical data on efficacy for therapy of selected conditions. A common complaint we hear about AMP products is that when you filter out the marketing material, not enough relevant clinical data exists in many cases.
 We’ve noted the basic rules of AMP regulations here, but in closing, we also need to say something about enforcement. The U.S. states have various independent regulatory requirements that require varying compliances, however the FDA is the primary enforcer of these rules. Because premarket approval is not required for a 361, a threshold barrier of product review does not exist. This puts a tremendous burden on the manufacturer, the marketer, and the prescribing physician. Many companies are proactive and diligent in assuring the highest quality and compliance, however like anything in life, there are exceptions. A lot of money can be made in a market where it’s easier to ask for forgiveness, than ask for permission in advance. Questionable products, regulatory vagaries, and limited enforcement may account for the proliferation of unregulated stem cell clinics across the country7. We fear that the proliferation of AMP injectables will prompt the next exposé. So until we see substantive change, be careful out there.
We’ve noted the basic rules of AMP regulations here, but in closing, we also need to say something about enforcement. The U.S. states have various independent regulatory requirements that require varying compliances, however the FDA is the primary enforcer of these rules. Because premarket approval is not required for a 361, a threshold barrier of product review does not exist. This puts a tremendous burden on the manufacturer, the marketer, and the prescribing physician. Many companies are proactive and diligent in assuring the highest quality and compliance, however like anything in life, there are exceptions. A lot of money can be made in a market where it’s easier to ask for forgiveness, than ask for permission in advance. Questionable products, regulatory vagaries, and limited enforcement may account for the proliferation of unregulated stem cell clinics across the country7. We fear that the proliferation of AMP injectables will prompt the next exposé. So until we see substantive change, be careful out there.
References:
- Cord Blood and the FDA - Mahendra Rao, MD PhD - Parent’s Guide to Cord Blood Foundation newsletter Jan 2015
- The ISSCR Guide to Stem Cell Treatments - International Society Stem Cell Research. Last updated 2023-06
- IOF poster - Dustin R. Berger, Nicolette F. Lyons, & Neven J. Steinmetz, presented 23 Oct. 2015 at IOF conference, In Vitro Evaluation of Injectable, Placental Tissue-Derived Products for Interventional Orthopedics
- IOF press release - 16 Dec. 2016, Interventional Orthopedics Foundation Shines Light on False Claims about Stem Cell Products
- IOF blog - 5 Jan. 2016, Companies Respond to IOF Findings Related to Placental Tissue-Derived Products
- Amniotic Stem Cell Challenge and YouTube featuring Dr. Centeno
- The Washington Post - Laurie McGinley 30 June 2016, Unregulated stem-cell clinics are proliferating across the United States


