You are here
What dose of cord tissue MSCs are needed for a treatment?
Therapies with mesenchymal stromal cells (MSC) from all sources are still under research, so the optimum dose has not been established for any diagnosis.
As of August 2020, only two papers have been published that summarized the MSC cell doses used in clinical trials. The first paper, by Couto et al. 2019, looked at the first decade of clinical trials with MSC from umbilical cord tissue (UC-MSC), over the years 2007-2017, and collected trials from everywhere in the world. The trials were divided between those that gave local injections of UC-MSC versus systemic administration into the blood stream. Their figure 4 showing number of trials versus total cell dose is repeated below. Please note that there are breaks in the dose axis to accommodate the very wide range of cell doses.
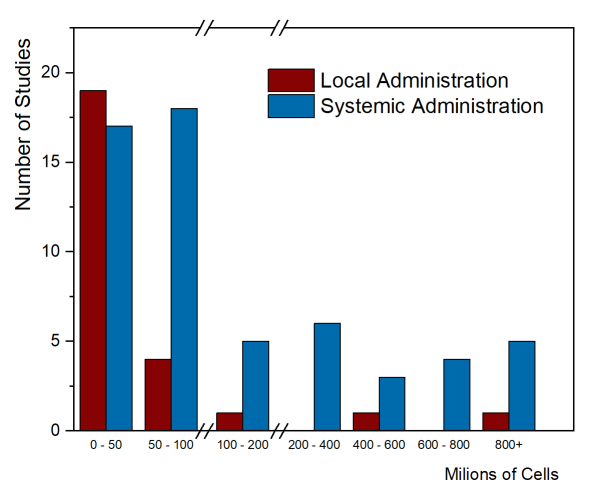
Clinical applications that employ systemic administration tend to have higher cell doses. It is not possible to give large doses locally, such as injecting a single arthritic joint or injecting the spinal cord. The few trials that achieved high total cell dose with local administration were trials that gave multiple doses. In this graph, the median total dose of the studies with local administration was 18.75 million cells, while the median total dose of the systemic administration studies was 80 million cells.
A second paper published by Kabat et al. 2020 did the same type of analysis with a different data set of clinical trials. They looked at clinical trials with any type of MSC (from bone marrow, fat tissue, umbilical cord tissue, etc.), over the years 2004-2018, but only the trials registered in the United States. Within their data, they divided the route of administration into six different groups. They looked at route of administration versus trial phase, cell dose, and patient diagnosis (see their figures 5A – 5C). The most common method of delivery was intravenous (systemic) administration and the median dose by that route was 100 million cells.
References
- Couto PS, Shatirishvili G, Bersenev A, and Verter F. 2019; Regenerative Medicine 14(4):309-319. First decade of clinical trials and published studies with mesenchymal stromal cells from from umbilical cord tissue
- Kabat M, Bobkov I, Kumar S, Grumet M. 2020; Stem Cells Translational Medicine 9(1):17-27. Trends in mesenchymal stem cell clinical trials 2004‐2018: Is efficacy optimal in a narrow dose range?
