You are here
Asymmetrex Technology to Count Stem Cells
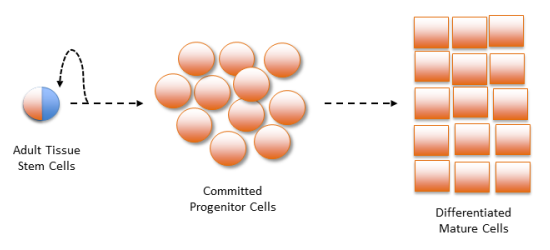 The perennial problem with counting adult tissue stem cells is well known among experts. At the root of the problem are the biological properties unique to stem cells: Stem cells have very low concentration in tissues of the body; stem cells divide to produce both more stem cells but also committed progenitor cells; and it is difficult to distinguish the stem cells from the committed progenitor cells.1-4
The perennial problem with counting adult tissue stem cells is well known among experts. At the root of the problem are the biological properties unique to stem cells: Stem cells have very low concentration in tissues of the body; stem cells divide to produce both more stem cells but also committed progenitor cells; and it is difficult to distinguish the stem cells from the committed progenitor cells.1-4
First, the concentration of stem cells in human tissues ranges from 1 in 1000 to less than 1 in 10,000. Methods that seek to isolate stem cells by growing them out in tissue cultures (explant methods) always produce a heterogeneous cell mixture, they are never pure stem cells. Even the most stem cell-enriched cultures only achieve a final stem cell concentration of a few percent.
Second, early committed progenitor cells are the immediate products of stem cell renewal by division. Unlike stem cells, progenitor cells cannot renew themselves indefinitely. Progenitor cells are committed to multiplying themselves for a limited number of divisions, before their lineages end with mature tissue cells that perform specific organ and tissue functions.5
Finally, until now there has been no practical method to distinguish stem cells from early committed progenitor cells. For example, all of the so-called “stem cell biomarkers” that are used to count stem cells (e.g., CD34, CD133, CD90) are expressed on the cell surface of both stem cells and committed progenitor cells.1,4 Since committed progenitor cells outnumber stem cells by as much as 100 to 1, they obscure all attempts to count stem cells by using these biomarkers. Another example of a measure that counts both stem and committed progenitor cells is the widely used colony-forming unit (CFU) assay6, which cannot discern whether a cell colony originated from a stem cell or from an early committed progenitor cell.
Asymmetrex provides Kinetic Stem Cell (KSC) counting technology that answers the crucial question of how to quantify tissue stem cells independent of committed progenitor cells. The underlying biological principle for the method is the asymmetric kinetics of stem cell self-renewal.7-9
Kinetic Stem Cell counting can be thought of as having two steps. The first step is to derive the algorithm for a specified combination of adult stem cell type and culture conditions. This step is also called the TORTOISE TestTM and takes about 3 weeks. In addition to revealing the count of stem cells, the algorithm also reveals insights about the rate of growth and viability of the committed progenitor cells. These insights could help a manufacturer to improve their therapeutic stem cell product and optimize their culture conditions.
Once the algorithm has been derived for a specified combination of adult stem cell type and culture conditions, it is possible to perform rapid counts of the stem cell number in new cultures within a mere 3 days. This is called the RABBIT TestTM.
Asymmetrex has reported numerous validations of Kinetic Stem Cell counting.7-9 The technology has been used to count stem cells from various human tissues, including liver and lung, to count hematopoietic stem cells from bone marrow and umbilical cord blood, and to count mesenchymal stem cells from bone marrow, umbilical cord tissue, and amniotic membrane. The technology is able to quantify stem cell fractions ranging from a few percent to as low as 1 in 10,000. Additional validations have explored the effects of stem cell-toxic agents and stem cell-activating agents.
The only alternative counting technology that exists for adult stem cells is the limiting-dilution SCID-mouse repopulating cell assay, which has been the gold standard for counting hematopoietic stem cells.11 Like Kinetic Stem Cell counting, the mouse assay also relies on statistical modeling, but requires animal testing and takes 16 weeks. Kinetic Stem Cell counting has the advantages of simplicity, low expense, greatly increased throughput, robustness, and application for other types of stem cells.
At present, Kinetic Stem Cell counting is available as a contract service. The assay can be applied to a testing segment of the stem cell therapy, to infer the stem cell dose of the entire unit. If the stem cell therapy goes through a manufacturing process that involves serial expansions of cultures, the software can provide stem cell count data at each step of the production process. This knowledge enables the manufacturer to optimize the process design. Asymmetrex continuously invites collaboration with stakeholders in the stem cell industry, including stem cell transplant centers, stem cell therapy companies, and academic stem cell labs. The sooner this stem cell counting technology is widely adopted, the sooner we can advance stem cell dosing in the practice of medicine.
References
- Ivanovic Z. (2010) Hematopoietic Stem Cells in Research and Clinical Applications: The “CD34 issue,” World J Stem Cells 2, 18-23.
- Sherley, J. L. (2018) Achieving Dose Standardization for the Stem Cell Clinical Trials Industry, Operations 10:03
- Sherley, J. L. (2018) The Stem Cell Emperor’s New Clothes, Analysis, Nov. 6
- Sherley, J. L. (2018) Dose Determination for Stem Cell Medicine: A Need Whose Time Has Come, in Perinatal Stem Cells: Research and Therapy, eds. A. Atala, C. Cetrulo, K. Cetrulo, S. V. Murphy, and R. Taghizadeh, Elsevier, (Amsterdam).
- Potten, C. S. and Morris, R. J. (1988) Epithelial Stem Cells In Vivo, J. Cell Sci. Suppl. 10, 45-62.
- Rich, I. N. (2015) Improving Quality and Potency Testing for Umbilical Cord Blood: A New Perspective, Stem Cells Trans. Med. 4, 967-973.
- Sherley, J. L. (2017) Methods for Determining the Effects of an Agent on Tissue Stem Cells, U.S. Patent No. 9,733,236.
- Sherley, J. L. (2018) Stem Cell Therapy: Resolving the Mismatch, Pharmaceutical Manufacturing, March, 38-41.
- Dutton R, Abdi F, Minnetyan L, Sherley JL. (2020) A Computational Simulation Technology for Specific Counting of Perinatal and Postnatal Human Tissue Stem Cells for Transplantation Medicine. OBM Transplantation 4(3):24.
- Paré, J.-F. and Sherley, J. L. (2006) Biological Principles for Ex Vivo Adult Stem Cell Expansion, in Current Topics in Developmental Biology, ed. G. Schatten, Elsevier, Inc. (San Diego), Vol. 73, 141-171.
- Ziegler, B. L. et al. (1999) KDR receptor: A Key Marker Defining Hematopoietic Stem Cells, Science 285, 1553-1558; DOI: 10.1126/science.285.5433.1553.
- Olsson, R. et al. (2013) Graft Failure in the Modern Era of Allogeneic Hematopoietic SCT, BMT 48, 537-543.
- Wagner, J. et al. (2014) One-Unit versus Two-Unit Cord-Blood Transplantation for Hematologic Cancers. N Engl J Med 371:1685-1694.



 James L. Sherley, MD PhD, is the founder and director of Massachusetts stem cell biotechnology company Asymmetrex, LLC. Asymmetrex develops and markets technologies for advancing stem cell medicine, including the first-in-kind technology for specific counting of adult tissue stem cells. This technology is also applied to design optimized procedures for more effective manufacturing of therapeutic adult tissue stem cells at greatly reduced cost. Dr. Sherley is a graduate of Harvard College, with a BA degree in biology, and the Johns Hopkins University School of Medicine, earning joint MD and PhD degrees. Prior to founding
James L. Sherley, MD PhD, is the founder and director of Massachusetts stem cell biotechnology company Asymmetrex, LLC. Asymmetrex develops and markets technologies for advancing stem cell medicine, including the first-in-kind technology for specific counting of adult tissue stem cells. This technology is also applied to design optimized procedures for more effective manufacturing of therapeutic adult tissue stem cells at greatly reduced cost. Dr. Sherley is a graduate of Harvard College, with a BA degree in biology, and the Johns Hopkins University School of Medicine, earning joint MD and PhD degrees. Prior to founding