You are here
Amniotic Membrane of the Placenta - Part 2
 Stem cell experts have proclaimed that allogeneic cells are the only way to make cell therapy viable as a commercial proposition for the treatment of the population at large. No one can argue that a ‘one-size-fits-all’ product enables commercialization, as particularly manifested through the economy of scale from higher volume production.
Stem cell experts have proclaimed that allogeneic cells are the only way to make cell therapy viable as a commercial proposition for the treatment of the population at large. No one can argue that a ‘one-size-fits-all’ product enables commercialization, as particularly manifested through the economy of scale from higher volume production.
However at AmnioChor, we believe that our ability to provide the highest quality health care must, and surely will, transcend the one-size-fits-all model as we move into the era of Precision Medicine and induced Pluripotent Stem Cells. We propose that the future of cellular therapy depends on the answers to these two questions:
- Is allogeneic the most efficacious cell therapy for the indication?
- Is allogeneic cell therapy our only business model to treat the population at large?
Over the years 2011-2015, the registry ClinicalTrials.gov recorded the launch of 211 clinical trials using perinatal cells for regenerative therapy, among them 143 trials with mesenchymal stem cells (MSC) from perinatal sources (Bersenev, Couto, & Verter, private communication). Our simplistic conclusion is that the current demand for perinatal MSC creates a need for biobanking capabilities that can serve all future therapeutic modalities.
The business mandate we envision is expanded biobanking of perinatal tissues and cells, both in private storage for family use and in research banks for the development of allogeneic therapies. One side of the business coin is the commercialization enabled by allogeneic therapies. But the flip side has to be our service to the autologous customer. We believe the best way to treat patients is to be able to provide autologous and matched allogeneic therapies along with off-the-shelf allogeneic products.
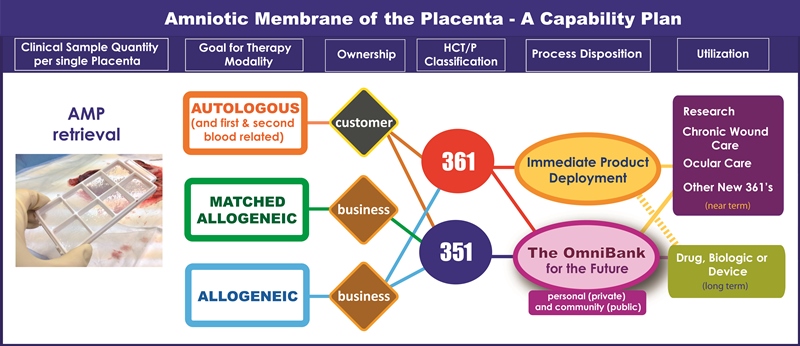
Figure 1 illustrates a capability model that what we seek to accomplish. When processed with our patented AmnioCeptTM technology, a single placenta yields multiple AMP samples that allow for multiple current and future therapeutic utilizations. We call this ‘Plurinatal’ Stem Cell technology.
But let’s put amnion into the big picture. To isolate the amniotic membrane, we need the placenta. Once we have the whole placenta, we can gain so much more than just the amnion. The human placenta is the absolute best source to secure a variety of valuable cells required as the fuel for new regenerative medicine and cellular therapies. We seek to utilize and preserve the healing power of the amnion tissue, plus the Amnion Epithelial Stem Cells (AESC’s) and the Amnion-derived Stem Cells (ADSC’s) within the placenta. The ADSC’s are MSC-like, and can easily be expanded in the laboratory. The placenta presents a prolific supply of AMP and multipotent stem cells.
Our strategic focus to provide three treatment modalities (autologous, matched allogeneic, allogeneic) requires the tactical ability to marry public and private biobanking models into what we call an ‘OmniBank’. From a business commercialization perspective, the economic models of today’s public and private cord blood banks are like two trains passing in opposite directions. Private cord blood banks are funded by customer fees, while public banks lack this valuable support mechanism and must charge high fees when a sample is released.
Like private cord blood banking, we believe there is a distinct patient/customer benefit derived from private biobanking of all perinatal tissues. We believe the demand for autologous protection will continue to increase with the evolution of therapies based on perinatal MSCs. Consequently, personal storage will continue to present a revenue opportunity for the business.
Like the public cord blood banking sector, the desire to provide off-the-shelf allogeneic products puts the ownership and the cost burden squarely on the bank. Nonetheless we believe the paradigm-shifter will be the multiple sample capacity and therapeutic versatility that can be achieved by banking AMP. We need to be capable of only using those cells that are needed today, while preserving all else for future therapies. In this way the OmniBank will be like a cellular version of the strategic petroleum reserve.
 We look forward to completing our discussion next month in Part 3: The Amniotic Membrane of the Placenta and Cures: What Can I Believe? - A Regulatory Summary.
We look forward to completing our discussion next month in Part 3: The Amniotic Membrane of the Placenta and Cures: What Can I Believe? - A Regulatory Summary.


