You are here
Cord Blood Therapy for Multiple Sclerosis
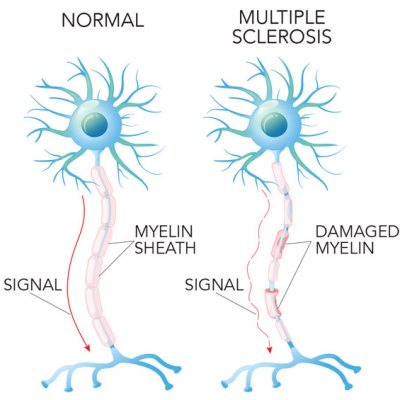 Multiple Sclerosis (MS) is an auto-immune disease where the patient’s immune system attacks the myelin sheaths that surround and protect nerve fibers in the central nervous system1. Areas of damaged myelin appear as lesions in MRI imaging of the brain and spinal cord. These lesions disrupt the nerve signals from the brain to other parts of the body. Symptoms can be highly variable, but usually include fatigue and impaired motor coordination. Nearly one million people in the United States are living with MS, and it is more common in women than men2.
Multiple Sclerosis (MS) is an auto-immune disease where the patient’s immune system attacks the myelin sheaths that surround and protect nerve fibers in the central nervous system1. Areas of damaged myelin appear as lesions in MRI imaging of the brain and spinal cord. These lesions disrupt the nerve signals from the brain to other parts of the body. Symptoms can be highly variable, but usually include fatigue and impaired motor coordination. Nearly one million people in the United States are living with MS, and it is more common in women than men2.
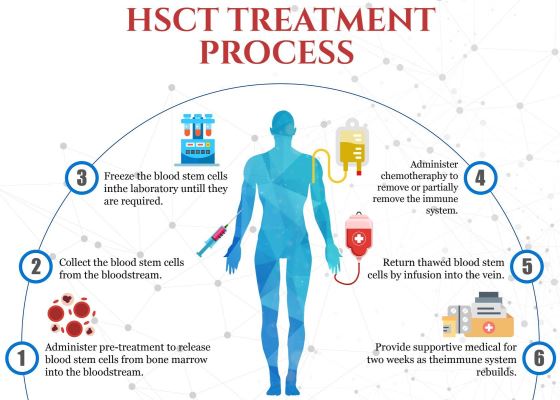
Transplants with “Hematopoietic”, or blood-forming, Stem Cells (HSCT) have become an accepted treatment for some forms of multiple sclerosis (MS). That is a big change from the last time we covered this disease in one of our 2016 newsletter articles3. At that time, a few clinical trials had published positive results for patients that underwent transplants with their own (autologous) bone marrow to treat relapsing-remitting MS. In one study at Northwestern University that enrolled 151 MS patients, 87% were progression-free four years later4. Analyses of the blood cells of MS patients that received HSCT have confirmed that the T-cell repertoire of their immune systems had been effectively reset to a less auto-reactive state5.
In October of 2020, the National Multiple Sclerosis Society of the United States officially recommended autologous HSCT for select groups of MS patients. This was later published in a special communication to the Journal of the American Medical Association6. The National Multiple Sclerosis Society says that patients with relapsing MS are candidates for autologous HSCT if they are: younger than age 50, disease duration less than 5-10 years, are still ambulatory, and they are experiencing flare ups of MS despite treatment with conventional therapy.
Actress Selma Blair revealed in August 2021 that she had undergone a stem cell transplant to treat a bad flare up of her MS, and she is now in remission7. A documentary about her life, Introducing Selma Blair, will air on the Discovery+ streaming platform October 21. That will probably lead to more famous people opening up about their struggles with MS.
It must be emphasized that HSCT has not become the standard treatment for MS at this time. The FDA has approved a number of drugs for MS that are considered to be highly effective “disease-modifying therapies” (DMT), and these are the first line of treatment8. Although a meta-analysis shows that HSCT are better than DMT at producing long-term remissions, this efficacy must be balanced against the risks of the HSCT6. To better understand which MS patients should be considered for HSCT, the National Institute of Allergy and Infectious Diseases is sponsoring a clinical trial titled “Best Available Therapy Versus Autologous Hematopoietic Stem Cell Transplant for Multiple Sclerosis (BEAT-MS)” which is currently recruiting at NCT04047628. One more advantage of HSCT for those patients that qualify is considerably lower cost than a lifetime of DMT drugs6.
The latest development in cell therapy for MS is the clinical trial NCT04943289 registered in June 2021 which plans to treat progressive MS with the cord blood product DUOC-01.
The research behind the use of DUOC-01 to treat MS goes back over a quarter of a century. In the 1990’s, Dr. Kurtzberg’s group at Duke University pioneered the use of cord blood transplants to treat children with inherited metabolic disorders. Certain inborn errors of metabolism called Leukodystrophies, including globoid leukodystrophy (Krabbe disease), metachromatic leukodystrophy, adrenoleukodystropy, and others, lead to abnormal myelination or loss of myelination in the central and peripheral nervous systems. Dr. Kurtzberg’s group was able to show that cord blood transplants could correct the metabolic defect in these patients, extend life, and in some cases, prevent progression of their disease9. Some of the children experienced measurable improvements in neurocognitive function9.
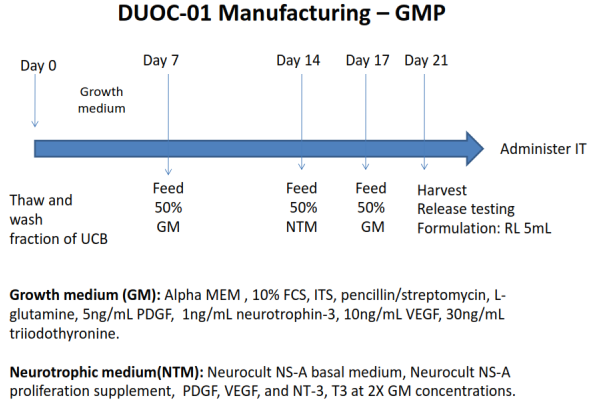
Subsequent research at Duke sought to identify the specific cells within cord blood that promoted remyelination of nerve sheaths. The active cells were shown to be derived from the CD14+ monocytes in cord blood. They could be grown in adherent cell culture into phagocytic cells that are able to stimulate oligodendrocytes, the cells that make myelin, to proliferate. In the early 2010’s Dr. Kurtzberg dubbed the active cells in cord blood “O-cells”, because they acted like oligodendrocytes. The product name “DUOC” was born as a combination of “Duke University” with “O-Cell”. However, subsequent research has described the cells as having “characteristics of macrophages and microglia”10-15. The process of manufacturing the DUOC-01 product takes three weeks. In 2014, clinical trial NCT02254863 began treating children diagnosed with demyelinating metabolic disorders using a combination of cord blood transplants plus DUOC-01. That trial is still recruiting.
The long-term goal of DUOC research has always been to develop a stand-alone cell therapy for adult demyelinating diseases. But many hurdles had to be surmounted before such a clinical trial could be launched. Two studies were conducted in animal models of MS to demonstrate that DUOC-01 accelerated remyelination, decreased gliosis, and reduced cellular infiltration in the brain16,17. The process of manufacturing DUOC cells has been improved incrementally. Currently the dose for each patient is manufactured from a single donated cord blood unit that is partially HLA matched. The Kurtzberg Lab is exploring modifications to the manufacturing process that will increase the number of DUOC cells that can be obtained from a single cord blood unit18, and exploring novel ways to measure remyelination19. The trial NCT04943289 of DUOC-01 for MS relies on intrathecal cell delivery, which is an injection into the cerebral-spinal fluid, and that has been proven safe by the prior trial NCT02254863 of DUOC-01 in children. It is very important to note that the DUOC-01 trial for MS targets patients with the primary progressive form of the disease, not the relapsing-remitting MS patient group which is eligible for autologous HSCT.
Not surprisingly, Duke University and the inventors of DUOC-01 have applied for a series of patents, the most recent filed in May 202115. Potentially, DUOC-01 could be applied to other degenerative conditions that cause demyelination of the Central Nervous System (CNS), as well as injury-induced CNS demyelination. The patent application explicitly covers Multiple Sclerosis, spinal cord injury, peripheral nerve damage, Parkinson's disease, Amyotrophic Lateral Sclerosis (ALS), and Alzheimer's disease.
Finally, DUOC-01 is a boon to public cord blood banks because it is manufactured from cryopreserved cord blood donations.
References
- National Multiple Sclerosis Society. What is MS? Accessed 2021-10-01
- National Multiple Sclerosis Society. Who gets MS? Accessed 2021-10-01
- Verter F. Stem Cell Therapies for AutoImmune Diseases such as Multiple Sclerosis. Parent's Guide to Cord Blood Foundation Newsletter Published 2016-04
- Burt RK, Balabanov R, Han X, Sharrack B, Morgan A, Quigley K, Yaung K, Helenowski IB, Jovanovic B, Spahovic D, Arnautovic I, Lee DC, Benefield BC, Futterer S, Oliveira MC, Burman J.Association of nonmyeloablative hematopoietic stem cell transplantation with neurological disability in patients with relapsing-remitting multiple sclerosis. JAMA 2015; 313(3):275-284.
- Muraro PA, Robins H, Malhotra S, Howell M, Phippard D, Desmarais C, Sousa AdPA, Griffith LM, Lim N, Nash RA, Turka LA. T cell repertoire following autologous stem cell transplantation for multiple sclerosis. J Clinical Investigation 2014; 124(3):1168-1172.
- Miller AE, Chitnis T, Cohen BA, Costello K, Sicotte NL, Stacom R, and the National Medical Advisory Committee of the National Multiple Sclerosis Society. Autologous Hematopoietic Stem Cell Transplant in Multiple Sclerosis. Recommendations of the National Multiple Sclerosis Society. JAMA Neurology 2021; 78(2):241-246.
- Mazziotta J, Dugan C. Selma Blair Is 'In Remission' from Her Multiple Sclerosis: 'My Prognosis Is Great'. People Magazine Published 2021-08-16
- National Multiple Sclerosis Society. Medications Accessed 2021-10-01
- Prasad VK, Mendizabal A, Parikh SH, Szabolcs P, Driscoll TA, Page K, Lakshminarayanan S, Allison J, Wood S, Semmel D, Escolar ML, Martin PL, Carter S, Kurtzberg J. Unrelated donor umbilical cord blood transplantation for inherited metabolic disorders in 159 pediatric patients from a single center: influence of cellular composition of the graft on transplantation outcomes. Blood 2008; 112(7):2979-2989
- Tracy E, Aldrink J, Panosian J, Beam D, Thacker J, Reese M, Kurtzberg J. Isolation of oligodendrocyte-like cells from human umbilical cord blood. Cytotherapy 2008; 10(5):518-d25.
- Tracy ET, Zhang CY, Gentry T, Shoulars KW, Kurtzberg J. Isolation and expansion of oligodendrocyte progenitor cells from cryopreserved human umbilical cord blood. Cytotherapy 2011; 13(6):722-729.
- Kurtzberg J, Buntz S, Gentry T, Noeldner P, Ozamiz A, Rusche B, Storms RW, Wollish A, Wenger DA, Balber AE. Preclinical characterization of DUOC-01, a cell therapy product derived from banked umbilical cord blood for use as an adjuvant to umbilical cord blood transplantation for treatment of inherited metabolic diseases. Cytotherapy 2015; 17(9):1314-1326.
- Saha A, Buntz S, Scotland P, Xu L, Noeldner P, Patel S, Wollish A, Gunaratne A, Gentry T, Troy J, Matsushima GK, Kurtzberg J, Balber AE. A cord blood monocyte-derived cell therapy product accelerates brain remyelination. JCI Insight 2016; 1(13):e86667.
- Scotland P, Buntz S, Noeldner P, Saha A, Gentry T, Kurtzberg J, Balber AE. Gene products promoting remyelination are up-regulated in a cell therapy product manufactured from banked human cord blood. Cytotherapy 2017; 19(6):771-782.
- COMPOSITIONS AND METHODS FOR THE TREATMENT OF DEMYELINATING CONDITIONS. Patent Application: 17/328,749 Filed: 2021-05-24 Publication: 2021-09-09
- Saha A, Buntz S, Patel S, Matsushima GK, Wollish A, Kurtzberg J, Balber A. DUOC-01, a candidate cell therapy product derived from banked cord blood, accelerates brain remyelination in NSG mice following cuprizone feeding. Cytotherapy 2014; 16(4):S61-S62.
- Saha A, Scotland P, Parrott R, ... Xu L, Filiano AJ, Kurtzberg J. DUOC-01, a cord blood derived cell therapy product, ameliorates experimental autoimmune encephalomyelitis, a murine model for multiple sclerosis. Cytotherapy ISCT Abstracts 2020; 22(5):S31-S32.
- Xu L, Parrott R, French M, Kurtzberg J, Filiano A. Optimizing the Production of a Human Umbilical Cord Blood-Derived Cell Therapy Product, DUOC-01. Stem Cells Translational Medicine 2021; 10(S1):S4.
- Zhang L, Ellor S, Sun JM, Liu C, Kurtzburg J, Song AW. DTI Tract-Based Quantitative Susceptibility Mapping: An Initial Feasibility Study to Investigate the Potential Role of Myelination in Brain Connectivity Change in Cerebral Palsy Patients During Autologous Cord Blood Cell Therapy Using a Rotationally-Invariant Quantitative Measure. J Magn Reson Imaging 2021; 53(1):251–258.


