Jesteś tutaj
Not all umbilical cord tissue banks are the same: Does storage method compromise parent access?
Awareness and popularity of cord blood banking has increased across Asia, the Americas, Europe and Australia since the first cord blood transplant in 1988 [1]. Cord blood is a rich source of haematopoietic stem cells (HSC). These specialised cells give rise to all other blood cells and are currently used to treat a range of blood disorders and immune system diseases including leukaemia, lymphoma, anaemia and other blood disorders [2, 3].
Most parents and grandparents exploring the idea of cord blood banking are not aware of the full potential of the umbilical cord tissue. In the last 10 years scientists have realised that the umbilical cord represents one of the most concentrated sources of Mesenchymal Stem Cells (MSC) [4]. Additionally, MSC derived from the umbilical cord are considered to be neonatal and exhibit significant physiological and biological advantages over MSC sourced from adults [5, 6].
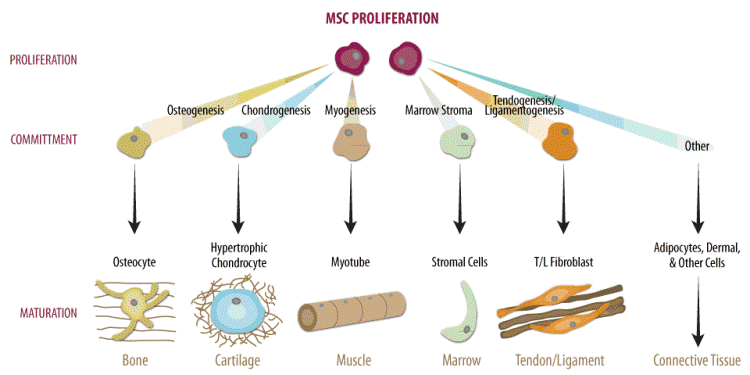
MSC have the ability to differentiate into cells of bone, cartilage, fat, muscle, tendons and ligaments (Figure 1) [7-9]. Unlike cord blood HSCs, which are mainly used as therapy for rare blood diseases, MSCs can contribute to therapies for osteoarthritis and sports injuries, problems which almost everyone experiences at some point in life. Due to their self-renewal ability, expansion potential, immunomodulation properties, and engraftment capacity, MSCs represent a promising candidate for cell based treatment of an array of medical conditions, such as, Alzheimer’s, cerebral palsy, Parkinson’s disease, psoriasis, rheumatoid arthritis, spinal cord injury, and stroke [9-21].
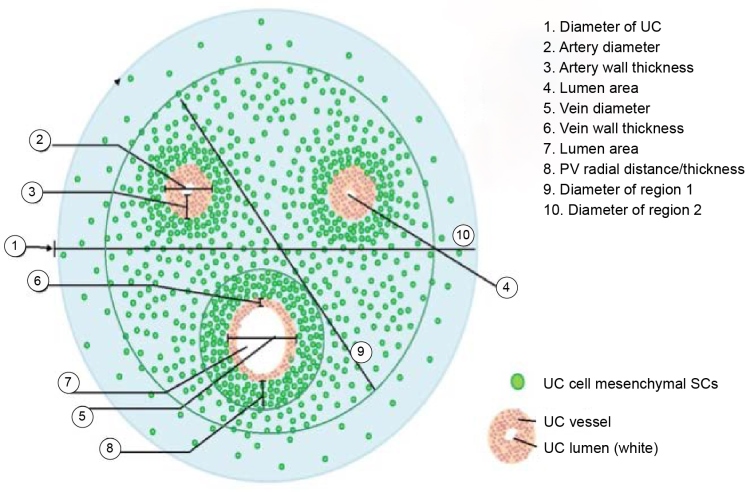
A closer examination of the tissue composing the umbilical cord reveals that the highest concentration of MSC is in the perivascular space surrounding the three blood vessels (Figure 2) [22]. A study at the University of Pittsburgh published in 2009 found that approximately 88% of all MSCs in the umbilical cord tissue are found in the perivascular tissue [22].
Despite the growing scientific appreciation of umbilical cord MSC as future therapeutic agents, the general public is still predominantly unaware that the optimal source of MSC is the umbilical cord. There is also a lack of education as to the best method to process and store these vital cells to ensure future access to MSC for therapy.
The Parent’s Guide to Cord Blood Foundation conducted a survey of family cord blood banks during the summer of 2015 [23]. At that time exactly half of the companies that operate their own laboratory were storing cord tissue in addition to cord blood. In response to the survey, about half of the companies that stored cord tissue admitted that they are storing whole or minced tissue, and not isolating MSC prior to storage [24]. In other words, storage of a tissue product, and not a cellular product, is the most common practice among banks that offer cord tissue storage to parents.
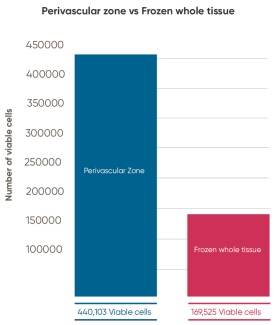 Cryosite, following patented protocols from Tissue Regeneration Therapeutics (TRT), isolates and stores the blood vessels of the umbilical cord, which includes the vital perivascular zone. Cryosite initiated a research project to compare the retrieval of MSC with their storage method versus the retrieval of MSC from whole cord tissue. The study consisted of a pairwise comparison of 9 umbilical cords which were processed by both methods. The number of viable nucleated cells isolated per gram of cord tissue was on average 0.44 million from the perivascular zone versus 0.17 million from whole cord tissue. This represents an average increase of 2.73 fold in viable cell density achieved by physically isolating perivascular zone tissue, a result that is strongly statistically significant (p-value of ≤ 0.01).
Cryosite, following patented protocols from Tissue Regeneration Therapeutics (TRT), isolates and stores the blood vessels of the umbilical cord, which includes the vital perivascular zone. Cryosite initiated a research project to compare the retrieval of MSC with their storage method versus the retrieval of MSC from whole cord tissue. The study consisted of a pairwise comparison of 9 umbilical cords which were processed by both methods. The number of viable nucleated cells isolated per gram of cord tissue was on average 0.44 million from the perivascular zone versus 0.17 million from whole cord tissue. This represents an average increase of 2.73 fold in viable cell density achieved by physically isolating perivascular zone tissue, a result that is strongly statistically significant (p-value of ≤ 0.01).
It is also important that the method used to cryopreserve cord tissue will allow viable MSC to be isolated and expanded when the product is thawed at a clinic [25]. Additional research by Cryosite tested 94 umbilical cords to determine if viable MSC could be retrieved from frozen perivascular tissue. All of the samples were successfully thawed and cultured to yield expanded MSC.
Finally, parents should be aware that in the event that they submit a request to release cord tissue or to isolate MSCs for therapy, they may face unforeseen difficulties if their storage bank did not have an appropriate license. Parents who are shopping for cord tissue storage may not realise that they need to be concerned about patent law. But in fact, those companies that discovered and developed umbilical cord tissue MSC sources (i.e. the perivascular space [26] and the sub-amniotic space [27]) own patents on methods of processing, preserving, and expanding these vital cells. It is a good idea for parents to ask a prospective cord tissue bank if they have licensed access to the technology needed to isolate and expand cells for therapeutic application. Therapeutic centres that one day might use cord tissue derived MSC may face legal issues if they accept MSC from cord tissue banks that have infringed patents. Clinical trials could also exclude cells that have not been collected by a licensed method.
In conclusion, parents and grandparents should be educated about all the factors that will influence their ability to access therapy with MSC from cord tissue, so that if they purchase this service they are making a fully informed decision.
References
- Gluckman, E. and V. Rocha, History of the clinical use of umbilical cord blood hematopoietic cells. Cytotherapy, 2005. 7(3):219-27.
- Hordyjewska, A., L. Popiolek, and A. Horecka, Characteristics of hematopoietic stem cells of umbilical cord blood. Cytotechnology, 2015. 67(3):387-96.
- Diseases Treated. Parent's Guide to Cord Blood Foundation [cited 2017].
- Sarugaser, R., et al., Human umbilical cord perivascular (HUCPV) cells: a source of mesenchymal progenitors. Stem Cells, 2005. 23(2): 220-9.
- Ishige, I., et al., Comparison of mesenchymal stem cells derived from arterial, venous, and Wharton's jelly explants of human umbilical cord. Int J Hematol, 2009. 90(2): 261-9.
- Harris, D.T., Umbilical cord tissue mesenchymal stem cells: characterization and clinical applications. Curr Stem Cell Res Ther, 2013. 8(5):394-9.
- Chan, A.S.H., et al. Improved expansion of MSC without loss of differentiation potential. [cited 2017].
- Ennis J et al. Isolation, characterization, and differentiation of human umbilical cord perivascular cells (HUCPVCs). Methods Cell BioI. 2008;86:121-136.
- Jackson, L., et al., Adult mesenchymal stem cells: differentiation potential and therapeutic applications. J Postgrad Med, 2007. 53(2):121-7.
- Wang, S., X. Qu, and R.C. Zhao, Clinical applications of mesenchymal stem cells. J Hematol Oncol, 2012. 5:19.
- Christodoulou, I., et al., Comparative Evaluation of Human Mesenchymal Stem Cells of Fetal (Wharton's Jelly) and Adult (Adipose Tissue) Origin during Prolonged In Vitro Expansion: Considerations for Cytotherapy. Stem Cells Int, 2013. 2013: p.246134.
- Phinney, D.G. and D.J. Prockop, Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair--current views. Stem Cells, 2007. 25(11):2896-902.
- Koc, O.N. and H.M. Lazarus, Mesenchymal stem cells: heading into the clinic. Bone Marrow Transplant, 2001. 27(3):235-9.
- Shohara, R., et al., Mesenchymal stromal cells of human umbilical cord Wharton's jelly accelerate wound healing by paracrine mechanisms. Cytotherapy, 2012. 14(10):1171-81.
- Salem, H.K. and C. Thiemermann, Mesenchymal stromal cells: current understanding and clinical status. Stem Cells, 2010. 28(3):585-96.
- Caplan, A.I. and D. Correa, The MSC: an injury drugstore. Cell Stem Cell, 2011. 9(1):11-15.
- Kean, T.J., et al., MSCs: Delivery Routes and Engraftment, Cell-Targeting Strategies, and Immune Modulation. Stem Cells Int, 2013. 2013: p.732742.
- Helmy, K.Y., et al., Stem cells and regenerative medicine: accomplishments to date and future promise. Ther Deliv, 2010. 1(5):693-705.
- Liu, Y., et al., Therapeutic potential of human umbilical cord mesenchymal stem cells in the treatment of rheumatoid arthritis. Arthritis Res Ther, 2010. 12(6): R210.
- Tsai, P.C., et al., The therapeutic potential of human umbilical mesenchymal stem cells from Wharton's jelly in the treatment of rat liver fibrosis. Liver Transpl, 2009. 15(5):484-95.
- Fan, C.G., Q.J. Zhang, and J.R. Zhou, Therapeutic potentials of mesenchymal stem cells derived from human umbilical cord. Stem Cell Rev, 2011. 7(1):195-207.
- Schugar, R.C., et al., High harvest yield, high expansion, and phenotype stability of CD146 mesenchymal stromal cells from whole primitive human umbilical cord tissue. J Biomed Biotechnol, 2009. 2009: p. 789526.
- Verter,F. and P. Silva Couto, Cord Blood Industry Report Parent's Guide to Cord Blood Foundation Nov.2015 [cited 2017]
- Silva Couto, P. and F. Verter, Survey of How Cord Blood Banks Process Cord Tissue. Transfusion, 2016. 56(6): ICBS1606
- Davies, J.E. Umbilical Cord Tissue Cells: Challenges and Promise. Parent's Guide to Cord Blood Foundation Feb.2017 [cited 2017].
- Ennis., J., Sarugaser, R., and Davies, J.E., Viable cells from frozen umbilical cord tissue, W.I.P. Organization, US Application Number 13630480 Granted 29.07.2014.
- Phan, T.T. and I.J. Lim, Isolation of stem/progenitor cells from amniotic membrane of umbilical cord, W.I.P. Organization, Publication Number WO/2006/019357, International Filing Date 03.06.2005.


