Вы здесь
Public Relations Tactics for Cord Blood Banks
The slides and discussion below are adapted from a presentation given during the 2025 AABB meeting. The learning objective of this presentation is: how can the cord blood community better communicate the therapeutic potential of cord blood, in the face of prevailing negative media bias?
Topics covered:
- Cord Blood has a serious image problem!
- Pubic Cord Blood Banks: Problems & Hope
- Family Cord Blood Banks: Problems & Hope
- Journalism: Theory
- Journalism: Practice
- How to handle a Hostile Interview
- Impact of Hostile Reporting
- How to respond to Hostile Reporting
- Avoid Cord Blood Cannibalism
- Key Take-Aways
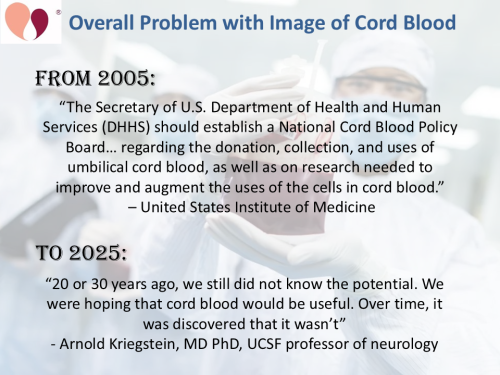
About twenty years ago, in the mid-2000s, “stem cells” were the hottest topic in medical research. Science magazines and news reporters were writing articles claiming that stem cells could cure whatever ails you. At that time, when a journalist approached a cord blood banker for an interview, it could be expected that the coverage would be positive. Part of the popularity of cord blood stem cells in those days was that they were seen as an “ethical alternative” to embryonic stem cells1. Currently, the hottest topic in medicine is the use of artificial intelligence (AI) to analyze data, and the halcyon days of enthusiastic reporting about stem cells from cord blood are only a memory.
Back in the 2000’s, the oncology community was very enthusiastic about relying on cord blood as a graft alternative for patients with minority HLA types who could not find a fully matched bone marrow donor. In 2005, the United States Institute of Medicine issued a book-length report advising the US Congress on the optimal structure for a National Cord Blood Program2. The rivalry for control of this program was so fierce that both the NMDP and the NY Blood Center each spent hundreds of thousands of dollars on lobbying campaigns3. The NMDP was awarded control of the program, and today it lives on as the C.W. Bill Young Cell Transplantation Program4.
These days, reporting sentiment has turned very negative towards cord blood banking. A key setback for the cord blood field came in 2020, when the Duke phase 2 clinical trial of cord blood for autism failed to meet its primary endpoints5. The on-line magazine VICE pounced on this topic and declared that Duke’s expanded access program was turning autism treatment into a “big business”6,7. This negative media coverage led to the shut-down of expanded access to cord blood therapy for autism in the United States, sending families seeking this therapy to other countries8. But that was not the end of the damage. Around that time, journalists started to realize that coverage describing cord blood banking as a “SCAM” could boost reader traffic. As a result, negative stories have become a trend, and more examples are provided below.
Sources for this slide:
- Anderson RT, Bottum J. Stem Cells: A Political History. First Things Nov. 2008 issue
- Institute of Medicine. 2005. Cord Blood: Establishing a National Hematopoietic Stem Cell Bank Program. Washington, DC: The National Academies Press.
- OpenSecrets.org
- Health Resources & Services Administration. Legislation. Last reviewed 2024-03
- Kurtzberg J. Results from the Duke ACT Study of Cord Blood for Autism: The Inside Scoop from Dr. Kurtzberg. Parent's Guide to Cord Blood Foundation Newsletter. Published 2020-07
- Merian A. A Controversial Autism Treatment Is About to Become a Very Big Business. VICE. Published 2021-10-06
- Kale S. Vice’s cunning, irreverent journalism is dead – and executives with bloated pay cheques helped kill it. The Guardian. Published 2024-02-27
- Verter F. Duke Removes Autism from Cord Blood Expanded Access. Parent's Guide to Cord Blood Foundation. Newsletter. Published 2023-06
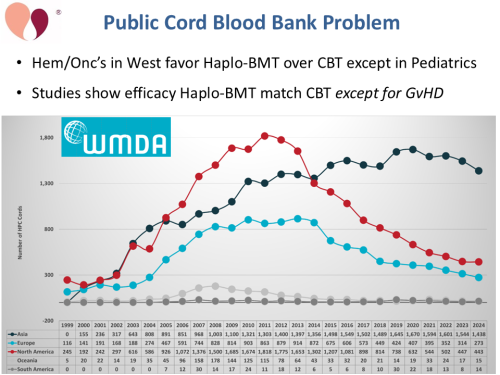
The problem facing public cord blood banks is well-known: many oncologists now prefer giving haploidentical (“haplo”) bone marrow transplants instead of cord blood transplants. As a result, the number of annual cord blood transplants performed in North America and Europe has fallen dramatically. This is usually illustrated with the above graph from the WMDA’s “Global Trend Report”1. The WMDA report drastically under-estimates the number of cord blood releases from public banks in China, but for those of us living in the western hemisphere that is slim comfort2.
Transplant oncologists may be influenced by pragmatic pressures for faster and less expensive therapies, but primarily they decide what treatment is best for their patients on the basis of studies in the medical literature. Several studies have been published about patients with myeloid leukemias which compared outcomes from transplants with either a single cord blood unit or an unmanipulated haplo transplant with post-transplant cyclophosphamide (PTCy). For large groups of leukemia patients, most outcomes are comparable between these two transplant methods. The one distinction is that cord blood transplants provide much lower chronic graft versus host disease, which is important for long term quality of life post-transplant3,4.
Hope for public banks comes from recent studies which have identified scenarios where transplant outcomes are much better if cord blood is incorporated into the treatment protocol. Hopefully, oncologists reading these studies will reconsider their treatment practices. A few important studies are briefly described here.
- For those patients with myeloid leukemias that are going into transplant testing positive for minimal residual disease (MRD), transplants with cord blood yield dramatically superior event-free survival over other grafts (50% vs 21%)5. To reiterate, while overall outcomes for the entire group of myeloid leukemia patients appear the same when they are transplanted with different graft sources, when those patients are stratified by MRD status it emerges that the MRD positive group does much better with cord blood3-6. It is believed that this result stems from the anti-leukemia effect of the T-cells in cord blood5,6.
- In China, several research groups are giving haplo-cord transplants rather than haplo or cord grafts alone. There are different groups using different treatment protocols for combining the cord and haplo grafts, and papers have been published which report on the diagnosis groups leukemia (AML and B-ALL), aplastic anemia, and thalassemia major7. Recently, the Lancet published a follow up to a multi-center phase 3 trial where patients with myeloid leukemia received either a haplo-cord transplant or a haplo transplant8. The outcomes of haplo-cord versus haplo were disease-free survival at one year 82.2% vs 65.6% (p=0.001) and overall survival at three years 80.5% vs 67.8% (p=0.013)7,8. This study showed better survival with haplo-cord transplants in all patient sub-groups, and a better safety profile for the haplo-cord transplants, with fewer complications causing treatment-related mortality.
- A phase 2 clinical trial treated 15 patients having various leukemias with a cord blood transplant plus an off-the-shelf product of non-HLA-matched NK cells expanded from cord blood (product Dilanubicel from Deverra Therapeutics)9. What is notable about this study is that at the one-year follow-up, the disease-free survival was 100% and the rates of acute or chronic graft versus host disease were zero9.
- Since slow engraftment is the main reason that oncologists avoid cord blood transplants, products have been developed which “expand” the hematopoietic stem cells in cord blood to speed engraftment10,11. Recently, two phase 2 trials treated 52 patients with high-risk leukemias with a single CBT expanded with the small molecule UM171 (product Zemcelpro from ExCellThera)12. Overall survival at two years was 63-67%, whereas the baseline survival for such high-risk patients is typically 30-34%12.
Finally, there is a public health aspect of cord blood transplants which is often over-looked by oncologists. The published papers only report on the outcomes of patients that were able to enroll in the studies. Oncologists tend to believe that in the United States there is a haplo donor “available” for nearly all patients13. But in fact, some patients face challenges accessing their treatment options. Patients from “underserved racial/ethnic populations” often do not receive prompt transplant work-ups, which leads to situations where they go to transplant later in the course of their disease, and they could benefit from a graft source like cord blood that is available quickly14.
Sources for this slide:
- WMDA Global Trends Report. Published 2025-09
- Verter F. Update on Cord Blood Utilization in China and Comparison to Other Countries. Parent's Guide to Cord Blood Foundation Newsletter. Published 2024-10
- Di Majo BE, … Ruggeri A. Single unrelated umbilical cord blood versus unmanipulated haploidentical HCT using PTCy in pediatric AML: a retrospective study on behalf of the EBMT PDWP and CTIWP. Bone Marrow Transplantation 2025; in press
- Konuma T, … Yanada M. Single Cord Blood Transplantation Versus Unmanipulated Haploidentical Transplantation for Adults with Acute Myeloid Leukemia in Complete Remission. Transplantation and Cellular Therapy. 2021; 27(4):334.e1-334.e11
- Horgan C, Mullanfiroze K, Rauthan A, Patrick K, Butt NA, Mirci-Danicar O, ... Wynn RF. T-cell replete cord transplants give superior outcomes in high-risk and relapsed/refractory pediatric myeloid malignancy. Blood Advances 2023; 7(10):2155-2165.
- Borrill R, Poulton K, Wynn R. Immunology of cord blood T-cells favors augmented disease response during clinical pediatric stem cell transplantation for acute leukemia. Frontiers Pediatric Immunology. 2023; 2023(11):3.1232281
- Verter F. Haplo-Cord Survival Better than Haplo Transplants Alone. Parent's Guide to Cord Blood Foundation Newsletter Published 2024-02
- Yu S, ... Liu Q. Haploidentical peripheral blood stem cells combined with bone marrow or unrelated cord blood as grafts for haematological malignancies: an open-label, multicentre, randomised, phase 3 trial. Lancet. 2025; 12(3):E190-E200.
- Milano F, ... Delaney C. Dilanubicel, a Non-HLA-Matched Pooled Universal Product, Transforming Single Cord Blood Transplantation Success Rates. Blood. 2024; 144(S1):377.
- FDA. BLA Approval Letter to Gamida Cell. Released 2023-04-17
- ExCellThera. Zemcelpro® (UM171 Cell Therapy) receives EC authorization as the first and only cell therapy for blood cancer patients without access to suitable donor cells. Press release. Published 2025-08-27
- Milano F, ... Cohen S. Infusion of UM171-Expanded Cord Blood Led to Excellent Survival in Patients with High-Risk Leukemias: Results from Two Independent Phase II Studies. Blood. 2023; 142(S1):1040.
- Fuchs EJ. Related haploidentical donors are a better choice than matched unrelated donors: Point. Blood Advances. 2017; 1(6):397-400.
- Fingrut WB, Scaradavou A, MD, Barker JN. Disparities in Allograft Access in the Era of Post-Transplant Cyclophosphamide-Based Mismatched Unrelated Donor Transplantation. J. Clinical Oncology. Letter to the Editor. Published 2024-11-06
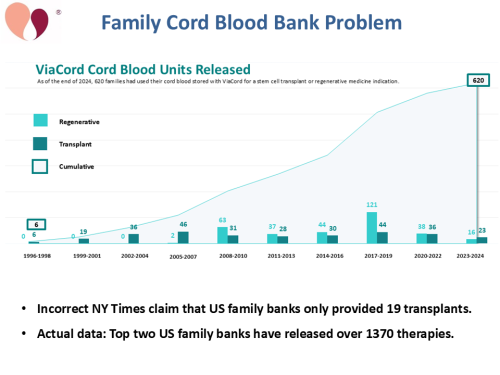
Unlike public banks, the rate at which family banks are releasing cord blood units for therapy has not significantly declined. As an example, we show data from the US bank ViaCord, which was selected because their graph displays both annual releases and cumulative releases on the same timeline1.
While back in the 2000’s, media coverage of stem cell research was very enthusiastic about early phase studies, now the tables are completely flipped and it can be hard to convince a reporter that the therapies released by family banks actually happened. We have encountered reporters who claim not to believe “self-reported” release numbers. For this reason, it is very important for family banks to publish as many patient testimonials as possible. When the audience sees a photo of the child, and an interview with the parents, it is hard to deny that the treatment took place.
In July 2024, the NYTimes reported that only 19 cord blood units from family banks had been used for transplants2. They obtained this number from the Center for International Blood and Marrow Transplant Research (CIBMTR) at the Medical College of Wisconsin. In fact, the two largest family banks in the US, CBR and ViaCord, have together released over 1370 cord blood units for therapy, including both traditional transplants and regenerative therapies3. When talking to reporters, cord blood bankers should emphasize that the therapies released from family banks are counted in peer-reviewed publications about clinical trials, and even therapies that were released to expanded access programs are counted in publications3-6.
Hope for family banks comes from an IPDMA statistical study that was published in the journal Pediatrics in April 2025. This ground-breaking study found that cord blood treatment for children with cerebral palsy gives them a statistically significant improvement in gross motor skills5. Indeed, compared to conventional therapies, the benefits of cord blood are “a large treatment effect”7. It is so large that experts in rehabilitation advise this option should be discussed when counseling the parents of children with cerebral palsy about their child’s treatment options7. The IPDMA statistics basically prove that cord blood therapy is effective for cerebral palsy. Unfortunately, this is not the same as an FDA approval, where the regulations still require running a phase 3 clinical trial. In the meantime, more countries are opening Compassionate Use programs to give cerebral palsy patients expanded access to this therapy8.
Our advice to family cord blood banks in the Western Hemisphere is to try to steer reporters away from interviewing oncologists and towards interviewing people who work with children that have cerebral palsy: both their parents and physical therapists. If the reporters talk to oncologists in the Western Hemisphere, they will come away with quotes about how cord blood is rarely used anymore for transplants. By comparison, in Asia there is a high public health burden from Thalassemia, and family banks often release sibling transplants to treat that diagnosis. Thus, in Asia it is much easier to make the case that cord blood in a family bank may be transplanted, and to present testimonials from sibling transplants. In the West it is necessary to rely more on the likelihood of applying cord blood for regenerative medicine treatments.
Sources for this slide:
- ViaCord. Over 620 cord blood units released by ViaCord®. Parent's Guide to Cord Blood Foundation Newsletter. Published 2025-07
- Kliff S, Ghorayshi A. Promised Cures, Tainted Cells: How Cord Blood Banks Mislead Parents. New York Times. Published 2024-07-15
- Verter F. Misinformation in New York Times article about Cord Blood Banking. Parent's Guide to Cord Blood Foundation Newsletter. Published 2024-08
- Dawson G, Sun JM, Baker J, ... Kurtzberg J. A Phase II Randomized Clinical Trial of the Safety and Efficacy of Intravenous Umbilical Cord Blood Infusion for Treatment of Children with Autism Spectrum Disorder. J of Pediatrics. 2020; 222:164-173
- Finch-Edmondson M, Paton M, Webb A, Ashrafi M, Blatch-Williams R, Cox CJ, ... Novak I. Cord Blood Treatment for Children With Cerebral Palsy: Individual Participant Data Meta-Analysis. Pediatrics. 2025; 155(5):e2024068999.
- McLaughlin C, West T, Hollowell R, Skergan ... Kurtzberg J. Expanded Access Protocol of Umbilical Cord Blood Infusion for Children with Neurological Conditions: An Update. Stem Cells Translational Medicine. 2021; 10(S1):S7–S8.
- Rosenbaum P, Palisano R. Cord Blood Treatment for Children With Cerebral Palsy (Commentary). Pediatrics. 2025; 155(5):e2024070467
- Verter F. Australia Allows Umbilical Cord Blood Therapy for Cerebral Palsy. Parent's Guide to Cord Blood Foundation Newsletter. Published 2025-06
It has been said that the heart of journalism is telling a story that some one does not want told. Various versions of this quote have appeared for over a century, and have been attributed to various sources, including George Orwell and William Randolph Hearst, among others1.
Journalists are supposed to follow a professional code of ethics2. If a journalist approaches you for an interview, and you ask them if they are planning to create a story that is an exposé about the cord blood industry, they should give you an honest answer. The professional code of ethics also requires journalists to “Respond quickly to questions about accuracy, clarity and fairness.” And “Acknowledge mistakes and correct them promptly and prominently.”
In journalism, there is always a tension between following the facts wherever they may lead, versus trying to extract a compelling story from the facts. Stories that somehow incorporate an abuse of power or a betrayal of trust are more compelling, so it is no wonder that journalists try to cast their articles as exposés. But when facts are cherry-picked for dramatic effect, then “investigative journalism” becomes “sensationalism”.
Sources for this slide:
- Quote Investigator. Quote Origin: News Is What Somebody Does Not Want You To Print. All the Rest Is Advertising. QuoteInvestigator.com Published 2013-01-20
- Society of Professional Journalists. Code of Ethics. Last revised 2014-09-06
In practice, the competition to get readers to click on an article or a podcast and follow it through has led journalists to cast more and more of their stories as exposés. Recent coverage of cord blood banking has been entirely of the exposé type. It is not “investigative” journalism, because reporters know that they are going to write a negative story before they interview anyone. There is absolutely nothing you can tell them which will recast that exposé as science reporting about the power of cord blood. You could tell them that, in your lab, cord blood has raised the dead, and they will still produce a negative story.
Most of the current problems faced by family cord blood banks stem from a very negative article published by the NYTimes in July 2024: “Promised Cures, Tainted Cells: How Cord Blood Banks Mislead Parents”1,2. Since then, multiple journalists at other news media outlets have been copying that article. Most recently, a podcast on Radio Canada copied the NYTimes content, transferred to the situation in Canada3. The NYTimes focused on four families who stored cord blood privately in the US, but then could not use the cord blood to treat their children because it was contaminated. This is a genuine tragedy, but the article makes it sound as if all private cord blood units are contaminated. Studies have shown that the contamination rate for cord blood units collected by obstetricians is under 10%4. By over-generalizing instances of contamination, the NYTimes created sensationalism.
There is a huge irony behind the NYTimes reporting, which almost no one talks about. Among the four families that faced a crushing disappointment when they discovered their child’s cord blood was contaminated, every single one of those families wanted to use the cord blood to treat cerebral palsy or autism. So those families represent the groundswell of public desire to store cord blood for personalized regenerative medicine, the very medicine that the reporters dismiss as not being real because it is not FDA approved.
In an effort to correct misinformation in the July 2024 NYTimes article about cord blood banks, Joanne Kurtzberg, MD, wrote a letter to the paper as the head of the Cord Blood Association, and Frances Verter, PhD, submitted a rebuttal as the head of the Parent's Guide to Cord Blood Foundation. We were both ignored.
But – if a news outlet receives push back from a source that has social media clout, they may actually address the mistakes. Here is an example: In January 2025, National Public Radio (NPR) ran a story about the role of influencers in sharing the news5. One of the people they interviewed, V Spehar, was outraged. Spehar thought they were giving an interview on a completely different topic. Spehar took to their TikTok channel, @UnderTheDeskNews, to issue a 6-minute video complaint, and gave interviews with other news outlets slamming NPR6. The upshot was that NPR devoted an entire web page of 2800 words to explain how they handled that interview7. Why did this story merit such a strong correction? Because V Spehar has more than 3 million followers on TikTok.
Sources for this slide:
- Kliff S, Ghorayshi A. Promised Cures, Tainted Cells: How Cord Blood Banks Mislead Parents. New York Times. Published 2024-07-15
- Verter F. Misinformation in New York Times article about Cord Blood Banking. Parent's Guide to Cord Blood Foundation Newsletter. Published 2024-08
- MacDonald-Dupuis N. Le mirage des banques privées de cellules souches. Radio Canada. Published 2025-09-16
- Sun J, Allison J, McLaughlin C, Sledge L, Waters-Pick B, Wease S, Kurtzberg J. Differences in quality between privately and publicly banked umbilical cord blood units: a pilot study of autologous cord blood infusion in children with acquired neurologic disorders. Transfusion. 2010; 50(9):1980-1987.
- National Public Radio (NPR). How influencers are impacting journalism. Weekend Edition. Published 2025-01-04
- Wiggins C. V Spehar talks TikTok, possible ban, Donald Trump, NPR. The Advocate. Published 2025-01-09
- McBride K. A source was unhappy about their interview on NPR. NPR Public Editor. Published 2025-01-16
At this point we are going to change topics from describing how journalists work to describing how cord blood bankers should handle interviews. There is an area of public relations called media training which prepares company representatives to effectively communicate with the media1,2. As part of media training, you will participate in mock hostile interviews. It is literally like training for verbal combat. Your goal is to go back to your talking points no matter what is thrown at you. When you are asked a question, you can start to give a boring answer to the question, but then mid-way through you “pivot” and say exciting things about your brand message. Hopefully the reporter will use the exciting quotes that are about your message. It is critical not to let yourself be provoked into saying anything that you will regret having quoted. We believe that Key Opinion Leaders (KOLs) in the cord blood community should obtain media training to help them to deal with the rising wave of negative reporting about cord blood.
Sources for this slide:
- Indeed Editorial Team. What Is Media Training? Definition and Effective Tips. Indeed. Published 2025-06-09
- Adoni Media. Media training: What is it and why is it important? Adoni Media Training.
There are various types of negative news stories, ranging from undesirable truths, to misinformation, to deliberately fabricated fake news. It is important to beware that “a fast-moving lie can do more damage to a company’s reputation than a slow, careful truth can fix”1.
Business professors have conducted research on the impact that negative news can have on corporate reputation. In their experiments, they exposed participants to fake news about a company, and then measured their judgments about that company, as well as how they thought the other participants would react. The impact of fake news hinges on an important aspect of human psychology: “People’s opinions are often shaped less by their own point of view than their perception of what others believe—a phenomenon that behavioral psychologists call ‘social proof’2”. As a result, even when people know that bad news about a company is fake, it erodes their trust in that company.
Sources for this slide:
- Haack P. When Fake News Targets Your Company. Harvard Business Review Podcast. Published 2025-09-09
- Etter M, Haack P, Mariconda S, Pizzetti M. How to Counter Fake News. Harvard Business Review. Published 2025-09
These days, when social media amplifies every message, it is often not enough to respond to negative reporting with the traditional approach of correcting the facts and/or refusing to comment. If a story is gaining traction on social media, with growing engagement numbers, then it is causing ongoing reputational harm. You have to ask yourself if the story has crossed a visibility threshold that warrants a more active response.
Experts in media relations advise a more proactive approach to building and maintaining the reputation of a brand, and these steps should be implemented before any negative news occurs. A “brand” may be a corporate product or it may be a non-profit foundation. Here are three key tactics from the playbook of experts in media training.
- Build credibility1,2. Brands that have a reputation for transparency will have a deeper reserve of consumer trust to protect them from negative news. Brands can build credibility by posting FAQ about how they work, by undergoing independent audits and certifications, and by inviting stakeholders to visit their sites and observe their operations.
- Social Proof1,2. Brands should recruit “allies” that will stand by them on social media if they come under attack. Allies can be peers, experts, trusted partners, and key stakeholders. Remember, psychology research shows that your audience cares what other people think, so it is important to demonstrate that you have allies who are supporting you in times of challenge.
- Doing well by doing good3,4. Research has shown that brands which are socially responsible have better reputation and customer loyalty. Social responsibility emphasizes having ethical leadership, taking actions that positively impact society, and protecting the environment. It is critical that the brand’s social responsibility strategy should be authentic and integrated into the brand identity, or else it will be perceived as “greenwashing” and fake. There will always be some customers who only care about price, but when a business is “doing good” they will also “do well” because a segment of their customers will be loyal to their products.
Sources for this slide:
- Haack P. When Fake News Targets Your Company. Harvard Business Review Podcast. Published 2025-09-09
- Etter M, Haack P, Mariconda S, Pizzetti M. How to Counter Fake News. Harvard Business Review. Published 2025-09
- Sohn J. “Doing well by doing good”? There’s a framework for that. George Mason University. Published 2025-07-02
- Abratt R, Silva Quaye E, Kleyn N. Conscientious corporate brands: the roles of organisational purpose, organisational culture, brand authenticity and corporate social responsibility. J. Brand Management. 2025; 32:418–437.
We encourage everyone who works with cord blood to avoid what we call “cannibalism”. We define “cannibalism” as situations where members of the cord blood industry, or members of the wider community that relies on cord blood for therapies, make disparaging remarks about other members of the community. An obvious example is when public bankers talk trash about family banks and say they are a scam. The experts giving these interviews expect the general public to understand the distinction between public banks versus family banks. They fail to understand a basic rule of public communication, which is that the public does not engage with news media in the same way as scientists1. Most likely, the only thing that a person in the street will remember from these negative quotes is that cord blood is somehow associated with a SCAM. By trash talking one segment of the community, the public bankers will end up hurting themselves. As experts in media messaging have said, even if negative news affects a competitor... “it might actually create a negative industry spillover”2.
A more thorny problem is how to deal with oncologists who indulge in insulting comments about family cord blood banks. Since very few oncologists are currently using cord blood, they probably do not care about the harm that their remarks can cause to the industry of cord blood banking as a whole.
An example of an attack on family banks which will have unintended consequences for public banks took place over the summer of 2025 in Canada. Radio Canada interviewed many cell therapy professionals in the Montréal area to produce a Canada-centric version of the 2024 NYTimes article. So far, as of Nov. 2025, this podcast has only aired in French3. In this podcast, it was stressed that the cord blood units in family banks are “too small” to be useful. One mother interviewed for the podcast decided to throw away her privately stored cord blood.
The Radio Canada podcast may backfire spectacularly, because Montréal is home to the biotech company ExCellThera. This company has developed a product, Zemcelpro®, which uses the molecule UM171 to expand the stem cells in cord blood units. Zemcelpro® from ExCellThera is now conditionally authorized in Europe, and is widely expected to be approved in Canada in the near future4,5. The publicity around this product emphasizes its ability to transform “small” cord blood units into units that can be used for transplant. "By increasing the number of cells, UM171 makes the stem cells from a small unit of cord blood more effective"5. The contradiction is obvious. Last summer, the experts in Montréal told the public that small cord blood units are useless. Soon, they are going to tell the public that they have a product which makes small cord blood units useful. Professionals in cell therapy will know that there is a difference between a cord blood unit which is bit too small versus very small, but the general public will not appreciate that distinction. Basically, the experts who told parents that small units are useless are going to be “hoist with their own petard”, as an old saying goes6.
Sources for this slide:
- Nisbet MC, Mooney C. Framing Science. Science Magazine. 2007; 316(5821):56.
- Haack P. When Fake News Targets Your Company. Harvard Business Review Podcast. Published 2025-09-09
- MacDonald-Dupuis N. Le mirage des banques privées de cellules souches. Radio Canada. Published 2025-09-16
- ExCellThera. Zemcelpro® (UM171 Cell Therapy) receives EC authorization as the first and only cell therapy for blood cancer patients without access to suitable donor cells. Press release. Published 2025-08-27
- Heinrich J. UM171: first Europe, then Canada. Université de Montréal. Published 2025-08-27
- Wikipedia. Hoist with his own petard. Accessed 2025-11-01