Jesteś tutaj
How many clinical trials use cord blood or cord tissue?
There is now an update to this article dated August 2022.
Source | # Cumulative Trials | # Recruiting Trials |
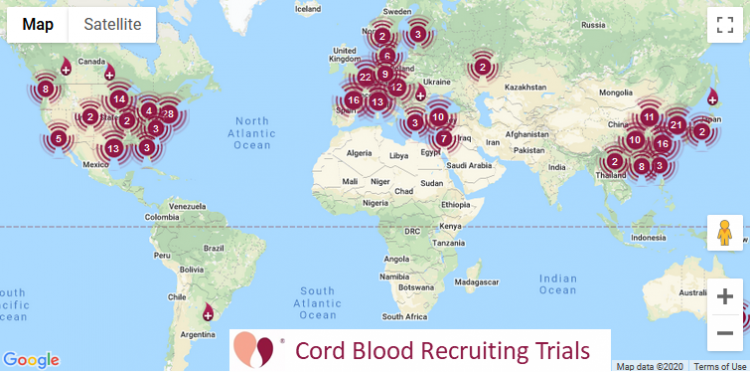
Cord Blood | 233 | 123 |
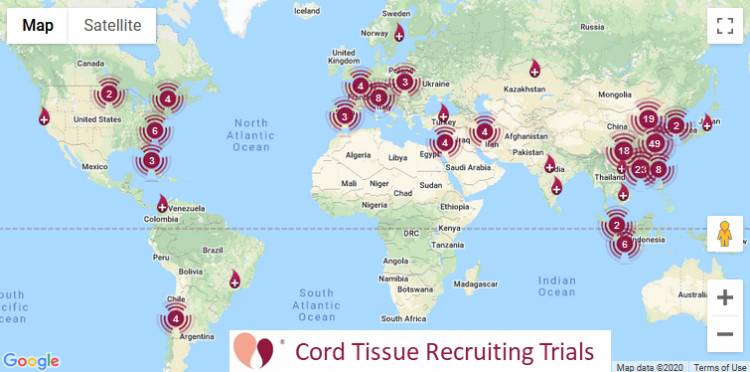
Cord Tissue | 291 | 155 |
Cord blood banks understandably like to advertise the number of clinical trials with cord blood or cord tissue. However, their numbers are often over-estimates. Many blogs claim that hundreds of trials have started, which side steps the fact that most of the early trials have already closed, and the number of trials that are currently running is smaller. Our goal in this article is to set the record straight, and provide accurate numbers that everyone can quote.
In order to add up the number of clinical trials that utilize cord blood or cord tissue, we have to explain where the trials are registered, how the trials are found, the different options for counting these trials, and check the current status of the trials.
Where clinical trials are registered
When researchers want to conduct a clinical trial, they must get approval from their national health authorities. Not surprisingly then, registries of clinical trials have been set up at the national level, with the first one launched in Japan in 1997. There are now over a dozen clinical trial registries in countries and regions around the world: see Table 1 of our 2017 paper. The most famous trial registry is the United States website ClinicalTrials.gov, and many trials that are actually taking place in other countries are also cross-listed to this registry. In the field of advanced cell therapy, our subsidiary CellTrials.org finds that averaged over the years 2018 and 2019, 66% of the world’s clinical trials can be found on ClinicalTrials.gov, while 34% can only be found on other registries.
How to find clinical trials
The process of compiling a list of clinical trials with a specific focus, for example all trials using cord blood, is very tedious. It requires running searches for keywords, like “cord blood”, in each registry. But that is only the first step. Just because a trial contains the words “cord” and “blood” does not mean that the trial actually uses cord blood for therapy. It is necessary to read every trial and pick out the ones that really utilize cord blood for therapy. Because trial compilation is labor intensive, a limited number of peer-reviewed publications have done this for the cell therapy field, and only four papers1-4 counted clinical trials with cord blood.
To illustrate how misinformation spreads: In early 2017, Dr. Verter sat in the audience of a conference, when a prominent oncologist from M.D. Anderson gave a talk and said “I checked ClinicalTrials.gov this morning, and there are 1365 trials with cord blood!” After the conference, our team at CellTrials.org took a deep dive into the data to check the accuracy of that claim. After weeks of work, we published a blog which found that: Clinicaltrials.gov had 1365 trial entries with the keywords “cord blood”, of which 425 were open trials about “cord” or “blood”, but only 209 were open trials with the exact phrase “cord blood”, and after scientific review of each trial, only 118 of the open trials were performing therapy with cord blood. Thus, only 118 of those 1365 hits (8.6%) were active cord blood trials.
What kinds of clinical trials use cord blood or cord tissue
Cord blood stem cells are used in two broad areas of medicine. Since the first cord blood transplant for Matt Farrow in 1988, cord blood has been used in over 40 thousand transplants worldwide for 80+ diseases. The use of cord blood for transplants is considered a “homologous” application of the stem cells, because the therapeutic stem cells perform their normal function of populating the immune system. The second application of cord blood stem cells is in advanced cell therapy, where the cells are either manipulated or performing a non-homologous activity. The most well-known example is regenerative medicine to treat neurologic conditions with cord blood, such as treatments for cerebral palsy or autism, and the first child treated was Abby Pell in 2005.
Umbilical cord tissue has only been used as a source for cell therapy since 2007, and all of the therapy falls into the category of advanced cell therapy. It is often assumed that the only active cell type found in cord tissue are the mesenchymal stromal cells (MSC) in the Wharton’s Jelly, but in fact a small number of trials use endothelial cells from blood vessels in the umbilical cord or epithelial cells from the lining of the umbilical cord.
Our numbers for cumulative clinical trials
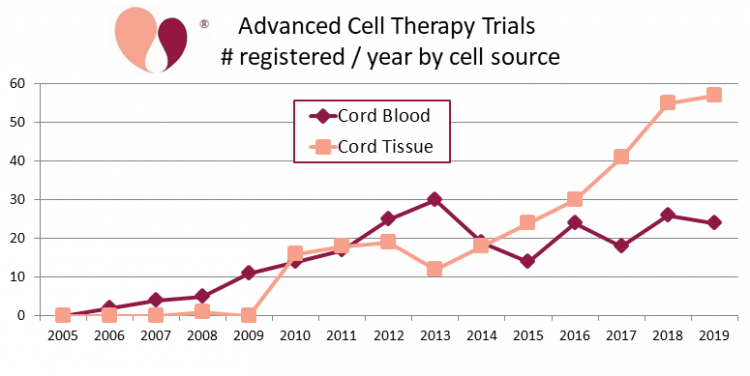
In 2017 we published the first complete compilation of clinical trials performing advanced cell therapy with perinatal stem cells, covering the years 2005-2015. Then in 2018 we published a follow up that extended the data through the end of 2017.
Even when focusing specifically on clinical trials that utilize perinatal stem cells, there are two different ways to count the trials: either by cell type or by cell source. In our previous publications we focused on cell type. Our papers discriminated between clinical trials that used MSC from perinatal sources (238 trials for 2005-2017), versus trials that used other cells from cord blood (131 trials for 2005-2017).
Now, for this article we revisited our data and divided the trials by the source of the cells. This time we combined all trials that obtained any cells from cord blood, whether the cells were MNC, MSC, oligodendrocytes, etc. We also included trials that used multiple cell types. Finally, to complete the table at the top of this article we added trials from our ongoing CellTrials.org data during the years 2018 and 2019. In total, the cumulative numbers of advanced cell therapy trials through the end of 2019 is 233 sourced from cord blood and 291 sourced from cord tissue.
Please note that our numbers for cumulative clinical trials only look at advanced cell therapy, because no one has compiled data for cord blood transplant trials going back to 1988.
Our numbers for currently recruiting clinical trials
The table at the top of this article also lists currently recruiting clinical trials. These numbers are quoted directly from the website ParentsGuideCordBlood.org, which has portals to search by diagnosis for recruiting trials that use cord blood or cord tissue. We compile these portals by checking the status of advanced cell therapy trials already archived at CellTrials.org, plus we run an additional search for clinical trials performing homologous cord blood transplants.
A word is needed about how we define “recruiting” trials for the purposes of the portals. We include trials that have status “recruiting”, “expanded access”, “invitation only”, and we include “not yet recruiting” for trials registered within the past two years. Once a trial is more than two years old and still shows status “not yet recruiting” we remove it, because we assume that either the researchers are not going to pursue the trial or they have finished it but never changed the status. We exclude trials that have status “active, but not recruiting”, “completed”, “terminated”, or “withdrawn”.
Is the field of therapy with newborn stem cells growing?
When we first started our portals of recruiting clinical trials in 2017, we had 114 cord blood trials and 49 cord tissue trials. As of 30 April 2020, we have 123 recruiting cord blood trials, a small increase that falls within the year to year variation in cord blood trial registrations. At the same time, the number of recruiting cord tissue trials has more than tripled to 155. We conclude that clinical applications with cord blood are holding steady, while the clinical use of cord tissue is on a growth trajectory.
References
- 1. Iafolla MA, Tay J, Allan DS. Transplantation of Umbilical Cord Blood–Derived Cells for Novel Indications in Regenerative Therapy or Immune Modulation: A Scoping Review of Clinical Studies. Biol. Blood Marrow Transplant. 2014; 20(1):20-25<
- 2. Couto PS, Bersenev A, Verter F. The first decade of advanced cell therapy clinical trials using perinatal cells (2005–2015). Regenerative Medicine 2017; 12(8):953-968.
- 3. Rizk M et al. Cell-Based Therapy Using Umbilical Cord Blood for Novel Indications in Regenerative Therapy and Immune Modulation: An Updated Systematic Scoping Review of the Literature. Biol Blood Marrow Transplant. 2017; 23(10):1607-1613
- 4. Verter F, Couto PS, Bersenev A. A dozen years of clinical trials performing advanced cell therapy with perinatal cells. Future Science 2018; 4(10):Editorial