Usted está aquí
Studying Cord Blood to Prevent Childhood Leukemia
 The concept that genetic screening of newborn blood could be used to predict which babies are at risk of childhood leukemia sounds almost like science fiction. In fact, this project is being spearheaded by leaders in childhood cancer research, and is currently funded by a five-year grant from NIH for a project called ReCord (NCT05014165).
The concept that genetic screening of newborn blood could be used to predict which babies are at risk of childhood leukemia sounds almost like science fiction. In fact, this project is being spearheaded by leaders in childhood cancer research, and is currently funded by a five-year grant from NIH for a project called ReCord (NCT05014165).
It has been known for over a century that if one identical twin develops childhood cancer, the other twin will often develop the same cancer. This led to the hypothesis that childhood cancers are triggered by mutations that develop in utero. In 1998, Professor Sir Mel Greaves proved this theory by demonstrating that identical genetic mutations appeared in sets of identical twins that both developed cancer1. He was knighted for this contribution to medicine2. Since then, multiple studies have shown that identical twins which develop childhood cancer have the exact same genetic anomalies, even though the actual onset of cancer may differ by over a decade in age. This illustrates the relative roles of nature versus nurture: the original mutation in utero creates a predisposition to cancer, which is somehow triggered after birth.
Acute leukemia is the most common childhood cancer and remains one of the leading causes of death in children under 15 years of age3. “Prevention, therefore, remains the ultimate goal,” say the principal investigators of the ReCord study. They are Logan Spector, PhD, professor at the University of Minnesota and member of the Children’s Oncology Group (COG), as well as Adam de Smith, PhD in genetics and assistant professor at the University of Southern California.
Finding the genetic origins of childhood leukemia is often called “backtracking leukemia to birth”. We now know that about 70% of cases of acute childhood leukemia, both ALL and AML, are caused by chromosomal translocations that are present at birth3,4. Over two dozen genetic mutations that cause childhood leukemia have been identified4.
 The ReCord study is recruiting pediatric leukemia patients at COG treatment centers that have their cord blood in storage. The investigators want to compare a genetic analysis of the patient’s blood at the time of leukemia diagnosis versus their umbilical cord blood from birth. The ReCord study uses droplet digital PCR (ddPCR) to analyze the DNA of single cells. They seek to learn what cell line was the origin of the leukemia, and what secondary mutations and other changes occurred between the pre-leukemia cells in the umbilical cord blood versus the full-blown leukemia in the blood taken at diagnosis. If the study recruits 250 children diagnosed with leukemia that have stored cord blood, approximately 182 of those children should have a known genetic mutation. So far, almost all of the children recruited had units stored in private cord blood banks. Once the children join the ReCord study, the parents cancel their private storage contract, and their cord blood unit is moved to the study center.
The ReCord study is recruiting pediatric leukemia patients at COG treatment centers that have their cord blood in storage. The investigators want to compare a genetic analysis of the patient’s blood at the time of leukemia diagnosis versus their umbilical cord blood from birth. The ReCord study uses droplet digital PCR (ddPCR) to analyze the DNA of single cells. They seek to learn what cell line was the origin of the leukemia, and what secondary mutations and other changes occurred between the pre-leukemia cells in the umbilical cord blood versus the full-blown leukemia in the blood taken at diagnosis. If the study recruits 250 children diagnosed with leukemia that have stored cord blood, approximately 182 of those children should have a known genetic mutation. So far, almost all of the children recruited had units stored in private cord blood banks. Once the children join the ReCord study, the parents cancel their private storage contract, and their cord blood unit is moved to the study center.
The fact that we have a list of mutations that are known culprits for most childhood leukemias raises the hope that eventually children can be screened to predict who is at risk for cancer, and then their family can take steps to prevent the development of cancer4. But making that hope a reality requires a few advances in both the technology and application of genetic testing.
One difficulty with screening children for leukemia mutations is the challenge of detecting a mutation. The mutations might be infrequent and only occur in a tiny fraction of the child’s blood cells, like the proverbial needle in a haystack. This is not a problem when a sample of cord blood is available for testing. However, the majority of children do not have their cord blood stored at birth. In the United States, nearly all children have blood taken at birth for mandatory newborn health screening. The number of screening tests that are run varies from state to state, and the blood is kept on paper cards as dried blood spots. These spots hold at most 50 microliters of blood, and the sample may be degraded after being kept in storage conditions that are less than ideal4. Thus, the small size of the universal blood spots is a limitation on the ability to apply genetic testing as a health screen for the entire public.
Another difficulty with screening children for leukemia mutations is that we must first conduct background studies in large populations of healthy children to learn the baseline frequency of these mutations. If a leukemia mutation only occurs in children that develop leukemia, then that mutation can be considered predictive. But if a mutation occurs commonly among children, and only rarely leads to cancer, then that mutation only represents a predisposition, and is not a prediction. This knowledge is still valuable, because the children with the predisposing mutation can be tracked more carefully to learn what triggers a few of them to develop leukemia.
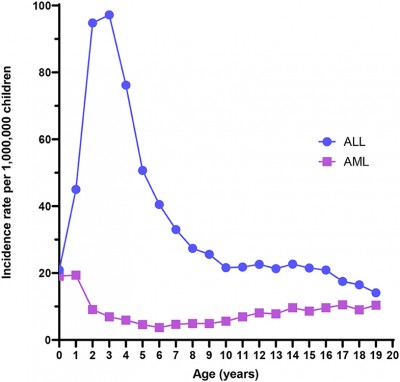
 A good example of the need for population baselines is the mutation called t(12;21)/ETV6-RUNX1, which is one of the most common causes of acute lymphoblastic leukemia (ALL) in young children. Early estimates of the prevalence of this mutation varied by a factor of 5004. Then, in 2018 a more accurate technology called Genomic inverse PCR for exploration of ligated breakpoints (GIPFEL) pinned down the prevalence of t(12;21)/ETV6-RUNX1 to 5% of healthy children5. This is a huge number; it is over a thousand times more common than the total incidence of ALL, which is only 42 cases per million children4. Clearly, the t(12;21)/ETV6-RUNX1 mutation is only a predisposition towards childhood leukemia, not a prediction for it. The other known mutations still need to be studied at the same level of detail to see if they are predictive.
A good example of the need for population baselines is the mutation called t(12;21)/ETV6-RUNX1, which is one of the most common causes of acute lymphoblastic leukemia (ALL) in young children. Early estimates of the prevalence of this mutation varied by a factor of 5004. Then, in 2018 a more accurate technology called Genomic inverse PCR for exploration of ligated breakpoints (GIPFEL) pinned down the prevalence of t(12;21)/ETV6-RUNX1 to 5% of healthy children5. This is a huge number; it is over a thousand times more common than the total incidence of ALL, which is only 42 cases per million children4. Clearly, the t(12;21)/ETV6-RUNX1 mutation is only a predisposition towards childhood leukemia, not a prediction for it. The other known mutations still need to be studied at the same level of detail to see if they are predictive.
In order to prevent childhood leukemia, we need to have a better understanding of what doctors call the “etiology” of pediatric cancer, which is to say how a genetic predisposition is triggered to become active disease4. The ReCord study will compare patient’s genetic analysis against known risk factors for leukemia and other information gleaned from parent surveys, in an effort to close the knowledge gap that exists between predisposition towards versus diagnosis with leukemia. Any future studies of the origins of childhood leukemia, for example a recent proposal that fetal blood cells evolve from two stem cell lines instead of one, will have to be consistent with the evidence from the ReCord study6.
The ultimate goal of the ReCord investigators is to develop genetic tests that can be applied as a public health screen. To meet this goal, the test must be accurate enough to identify at-risk children without generating lots of false positives, plus robust enough to be used on dried blood spots. If these goals can both be met, it would be a great advance for pediatric medicine and public health.
References
- Ford AM, Bennett CA, Price CM, Bruin MCA, Van Wering ER, Greaves M. Fetal origins of the TEL-AML1 fusion gene in identical twins with leukemia. PNAS 1998; 95(8):4584-4588.
- The Institute of Cancer Research (ICR). Pioneering scientist Mel Greaves is knighted after research to unveil cause of childhood leukaemia. News archive Published 2018-12-28
- NIH Reporter. Backtracking Leukemia-Typical Somatic Alterations in Cord Blood at Single-cell Resolution. Project Number 1R01CA262012-01
- Marcotte EL, Spector LG, Mendes-de-Almeida DP, Nelson HH. The Prenatal Origin of Childhood Leukemia: Potential Applications for Epidemiology and Newborn Screening. Frontiers Pediatrics 2021; 9:639479.
- Schäfer D, Olsen M, Lähnemann D, Stanulla M, Slany R, Schmiegelow K, Borkhardt A, Fischer U. Five percent of healthy newborns have an ETV6-RUNX1 fusion as revealed by DNA-based GIPFEL screening. Blood 2018; 131(7):821–826.
- Patel SH, Christodoulou C, Weinreb C, Yu Q, da Rocha EL, Pepe-Mooney BJ, Bowling S, Li L, Osorio FG, Daley GQ, Camargo FD. Lifelong multilineage contribution by embryonic-born blood progenitors. 2022; Nature Published online 2022-06-15


