Sie sind hier
7 Myths & Realities about Perinatal Stem Cells
März 2023
References
- Lucie. Stem Cells Illustration. BIO-Grafik
- Zbinden A, Canté-Barrett K, Pike-Overzet K, Staal FJT. Stem Cell-Based Disease Models for Inborn Errors of Immunity. Cells 2022; 11(1):108.
- Taghizadeh RR, & Sherley JL. Expanding the therapeutic potential of umbilical cord blood hematopoietic stem cells. Perinatal Stem Cells 2009; Chapter 2, Pages 21-35.
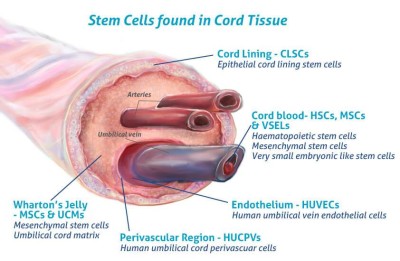
- Cells4Life. Umbilical Cord Tissue Stem Cells – more valuable than you think. Blog published 2016-08-05
- Dutton R, Abdi F, Minnetyan L, Sherley JL. (2020) A Computational Simulation Technology for Specific Counting of Perinatal and Postnatal Human Tissue Stem Cells for Transplantation Medicine. OBM Transplantation 4(3):24.
- Sherley JL. Kinetic Stem Cell Counting. Parent’s Guide to Cord Blood Foundation News Published 2022-06
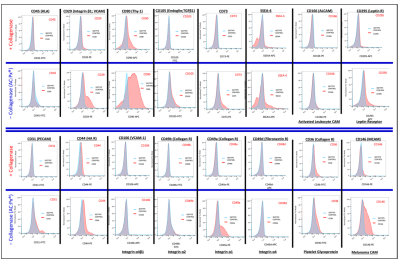
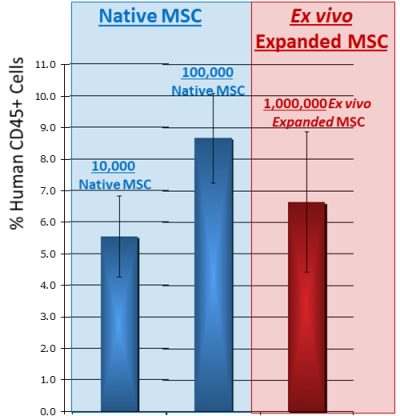
- Taghizadeh RR, Cetrulo KJ, Cetrulo CL. Collagenase Impacts the Quantity and Quality of Native Mesenchymal Stem/Stromal Cells Derived during Processing of Umbilical Cord Tissue. Cell Transplantation 2018; 27(1):181-193.
- Briddell R, et al. Blood 2011; 118(21):4398.
- Balci D & Can A. The assessment of cryopreservation conditions for human umbilical cord stroma-derived mesenchymal stem cells towards a potential use for stem cell banking. Curr. Stem Cell Res. Ther. 2013; 8(1):60-72.
- Moll, G. et al. Do cryopreserved mesenchymal stromal cells display impaired immunomodulatory and therapeutic properties? Stem Cells. 2014; 32:2430–42.
- Briddell R, Perinatal Stem Cells Isolated From Complete Umbilical Cord Tissue for Family Stem Cell Banking and Potential Therapeutic Use. Perinatal Stem Cells, Academic Press, 2018; Chapter 19, Pages 257-269.
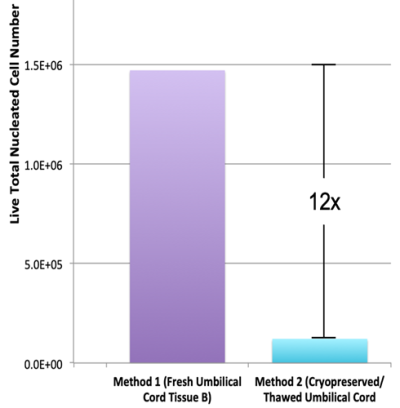
- Skiles ML, Marzan AJ, Brown KS, Shamonki JM. Comparison of umbilical cord tissue-derived mesenchymal stromal cells isolated from cryopreserved material and extracted by explantation and digestion methods utilizing a split manufacturing model. Cytotherapy 2020; 22(10):581-591.
- Cottle, C. et al. Impact of Cryopreservation and Freeze-Thawing on Therapeutic Properties of Mesenchymal Stromal/Stem Cells and Other Common Cellular Therapeutics. Current Stem Cell Reports 2022; 8(2):72-92.
- Taghizadeh RR, Cetrulo KJ, Cetrulo CL. Wharton's Jelly stem cells: Future clinical applications. Placenta 2011; 32(S4):S311-S315.
- Galipeau J & Sensébé L. Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell 2018; 22(6):824-833.