You are here
FAQs
Umbilical cord blood is one of the richest sources of stem cells found in nature. While cord blood stem cells are not embryonic, they are more pristine than the stem cells in adults, because they are younger and they have had less exposure to illness or environmental factors.
Most of the stem cells in cord blood are blood-forming stem cells, also known as “hematopoietic” stem cells or HSC. The presence of HSC is what enables cord blood transplants to be used as a substitute for bone marrow transplants. However cord blood transplants have advantages and disadvantages compared to stem cell transplants from adult donors. The main advantage of cord blood is that it does not have to be exactly matched to the patient like transplants from an adult donor. The main disadvantages are that it is hard to collect enough cord blood to transplant an adult, and cord blood stem cells are slower to engraft.
Cord blood also has applications in regenerative medicine. This is due to a combination of additional types of stem cells in cord blood, plus the fact that the cells in cord blood release chemicals that signal the body to heal itself. These chemicals are called cytokines and the cell-to-cell signaling is called the paracrine effect. Cord blood has been used in clinical trials around the world as therapy for infants with cerebral palsy and other brain injuries. Published studies have shown that cord blood stem cells benefit young children with neurologic injury, even though the mechanism of action is not yet fully understood. Additional clinical trials are applying cord blood to neurologic conditions in adults, including stroke, multiple sclerosis, and spinal cord injury.
Cord blood banking refers to the whole process of collecting and preserving the stem cells that remain in the blood of the umbilical cord and the placenta after the birth of a baby. Today, many private cord blood banks also offer storage of newborn stem cells from the tissue of the umbilical cord and/or the placenta.
Cord blood is in use today in hospitals around the world. There are over 80 diseases treated with cord blood as a standard of medical care, although admittedly many of these are rare disorders. Clinical trials with cord blood are developing therapies for more common childhood disorders. Cord blood can be donated to public banks for patients seeking donors, or stored in family banks for future use by the baby’s family.
Within the United States, healthcare providers can order shipments of our printed parent brochures for free in both English and Spanish. Parent's Guide to Cord Blood Foundation has developed educational brochures for parents that cover both public donation and private banking in a balanced way. Our brochures are available for download from cordbloodeducation.org in over a dozen languages and dialects, with the content adjusted to the national situation in different countries. These brochures were approved by our Scientific and Medical Advisory Panel and within the United States they are updated annually to adjust prices and include new clinical trials.
Our Foundation has also developed several Fact Sheets for medical professionals that are available for download from doctorsguidecordblood.org.
Health on the Net Foundation (HON) was an international non-profit under the United Nations dedicated to inspecting and accrediting medical websites for accurate and ethical content. Parent's Guide to Cord Blood Foundation was the only cord blood organization that was continuously accredited by HON, from May 2001 until HON shut down Dec. 2022
Websites accredited under the HON code are committed to abide by these 8 principles:
- Authority - Give qualifications of authors
- Complementarity - Information to support, not replace physician
- Confidentiality - Respect the privacy of site users
- Attribution - Cite the sources and dates of medical information
- Justifiability - Justification of claims / balanced and objective claims
- Transparency - Accessibility, provide valid contact details
- Financial disclosure - Provide details of funding
- Advertising - Clearly distinguish advertising from editorial content
We are the world's only organization dedicated to cord blood education that can claim all of the following:
- we are incorporated as a non-profit,
- we cover both public donation and private storage in a balanced way,
- our content is written by scientists,
- we are supervised by an Advisory Panel of leading scientists and physicians,
- and we are accredited by Health on the Net Foundation (HON).
Our website contains numerous unique cord blood resources:
- World's only complete searchable maps of family banks, public banks, FACT banks, AABB banks
- Within the United States, the only complete searchable map of hospitals accepting cord blood donations
2. Sterilize before every needle stick. When in doubt, sterilize again!
3. Volume, volume, volume. You want to "milk the cord" for as much blood as possible. If the blood vessel you are using stops working, try another or move upstream, but sterilize first.
The phrase "delayed cord clamping" means to wait before clamping the umbilical cord after birth.
Prior to birth, the baby relies on the umbilical cord blood for oxygen and nutrition. In the moments after birth, the baby takes its first breathe. At that moment of the first breathe, the baby's lungs call for more blood supply and some blood from the umbilical cord vein enters the baby. But at the same time, when the baby starts to breathe, the arteries that carry blood from the baby to the umbilical cord constrict. Hence the blood circuit between the baby and the placenta shuts down once the baby breathes. Some blood may continue to flow from the umbilical cord to the baby if uterine contractions put pressure on the placenta. Medical research shows that any time after the first breathe is an OK time to clamp the umbilical cord. We have a table of medical society recommendations for delayed cord clamping.
An important caveat is that, when a baby is born prematurely, the baby may not spontaneously breathe. In this case the baby will not spontaneously draw blood from the umbilical cord. We have a news article about cord milking as an alternative to delayed cord clamping, and whether it is safe for premature babies.
The median size of cord blood collections in family banks is 60mL or 2 ounces. That small volume of blood corresponds to 470 million Total Nucleated Cells (TNC) or 1.8 million cells that test positive for the stem cell marker CD34. Thus, most healthy full-term babies have over a million blood-forming stem cells for cord blood banking. By comparison, most public cord blood banks will only keep collections that are much bigger than average, and throw out the donations that are below a threshold of a billion TNC, corresponding to a blood volume of about 100 mL or 3 ounces.
Reference:
Sun, JJ et al., Transfusion Sept. 2010; 50(9):1980-1987 doi:10.1111/j.1537-2995.2010.02720.x
It literally only takes minutes to save the stem cells in cord blood. Once the umbilical cord is clamped, it is wiped with antiseptic and a needle is inserted into the vein in the umbilical cord to withdraw a few ounces of blood.
There are three methods of collection in common use. One is to hang a blood bag lower than the mother and let gravity draw blood down the tube into the bag. This method is used in most countries of the world, because it has the fewest steps, and therefore the fewest opportunities for mistakes or contamination.
The second method is to actively draw the blood out, just like when a person has a blood draw for a medical test. The draw can be done with a standard syringe or with a bulb in the tubing of the blood bag that creates suction. Studies have shown that actively drawing the blood will collect a larger volume faster.
Third, some banks collect cord blood "ex utero" which means "outside the uterus". They wait until the placenta is delivered, and then a trained technician takes it into another room and puts it on a high shelf so that all of the blood in the umbilical cord and some from the placenta can be drained.
If you live in the United States, we have a searchable map of all hospitals that accept cord blood donations. We are the only website that has a complete map and we work in partnership with the national donor registry Be The Match to update it quarterly.
In other countries we do not have this level of detailed information. Your best option is to look up the list of public banks in your country and contact them to find out where they accept donations.
No - cord blood that has already been stored in a family bank cannot be transfered to a public bank. When a public bank collects cord blood donations that will potentially be released to strangers, they are required to perform a very detailed family health history and collect a sample of the mother's blood. This screening must be performed at the time of donation and it cannot be retroactively performed for cord blood in a family bank.
An additional issue is that any given cord blood bank is very reluctant to trust the processing and storage of another bank. It is almost impossible to transfer cord blood from one bank to another, even among family banks. They do not want to accept a cord blood unit that was processed by another organization due to liability concerns.
There is no medical reason why parents should have to register for cord blood donation weeks in advance. Given adequate staffing and efficient management, a public cord blood bank could check the mother's health history and offer the family Informed Consent when they arrive at the hospital to give birth. Examples of public cord blood banks in the Untied States that accept walk-in donations are the NY Blood Center and the Cleveland Cord Blood Center.
However, most public cord blood banks in the United States do not have enough dedicated staff to handle donation requests, so in order to process them they require that mothers register weeks ahead of their due date. About five years ago the registration deadline was at 36 weeks, but as of 2016 the deadline is 34 weeks gestation.
Within the United States, as of 2022 only one program provides parents with a collection kit that they can take to the hospital and then send a mail-in donation to a public cord blood bank or a research laboratory.
- Cord for Life (800-869-8608) - accepts cord blood donations if you register by week 34
In the United States, about 80% of cord blood donations are discarded. The primary reason is that the collection volume is too small. Public banks exist to provide transplants for patients throughout the world, and hence they only store cord blood donations that are big enough to transplant a large child or small adult. On top of that medical requirement to only accept the bigger collections, in recent years public banks have raised their storage thresholds in response to economic pressures.
Reference:
Magalon et al. 2015 Banking or Bankrupting: Strategies for Sustaining the Economic Future of Public Cord Blood Banks PLOS ONE doi:10.1371/journal.pone.0143440
Health requirement: The donor registry Be The Match has a short pre-screening questionnaire where you can learn if you can donate cord blood. Before the actual donation, the mother would have to undergo a more detailed maternal health screening. Here is another article about the health requirements from our Foundation's newsletter.
Location requirement: Less than 200 hospitals in the United Sates have programs to accept cord blood donations, and they are all large birthing centers located in communities with racially diverse populations. Those parents who are not delivering at a hospital that accepts donations can try to register for a mail-in donation.
Timing requirement: Most donation programs require the mother to register in advance, typically by week 34 of the pregnancy (the due date is at 40 weeks). A few donation programs will sign up mothers during labor for permission to collect the cord blood, then if the collection qualifies for public banking they will go back to the mother before she leaves the hospital to get a full informed consent.
Although a few such cases have actually happened, it is very very unlikely to get your cord blood back once it is donated. Donating cord blood is not a way of banking for your family for free.
When a mother signs the Informed Consent form to donate cord blood, she gives up any guaranteed access to that blood. First of all, the public bank may throw the blood out simply because it does not meet their size threshold, or simply because the paperwork is not complete. Secondly, even if the blood does make it into public storage, it may be released to some one else.
Unlike organ donors, cord blood donors do not receive any priority treatment or waived fees if your child later needs a donor. The reward for donating cord blood is the possibility that your baby may Be The Match that saves a life.
Community Banking is a new sharing economy model of cord blood banking that was pioneered by LifeCell in India. Parents who choose to store their child's cord blood in a community bank will have access, in the event of medical need, to all of the other cord blood units in the community bank. A community bank is like a public cord blood bank in that the members are supporting each other, but it is also like a private bank because the members pay for this service and outsiders cannot participate.
Community banking can fill an unmet health need in a country like India, where there is no national network of public banks and the population has unique genetics that are not covered by banks elsewhere in the world. Scientists have calculated that a community bank holding 25,000 cord blood units would be able to match 96.4% of Indians close enough for a transplant.
Community banking is different from "hybrid" banking where both public and family banks share a laboratory, because in hybrid banks the pubic and family sides operate separately. In a community bank the public and family functions are blended.
Although several cord blood banks in India are licensed to conduct public banking, our research finds that for several years there have been virtually no cord blood transplants in India from public banks. The only banks providing donor (allogeneic) cord blood transplants in India right now are Community Banks.
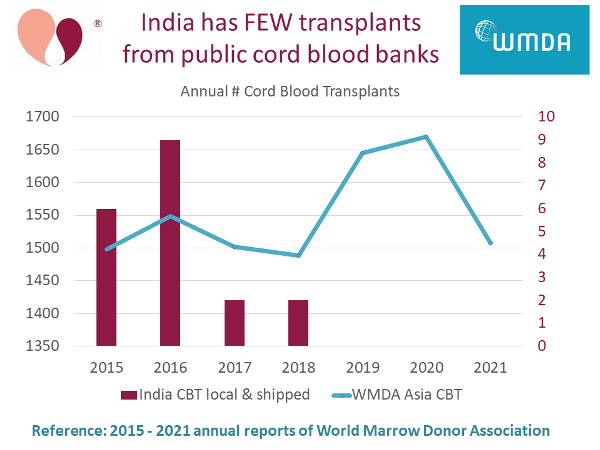
We have compiled this information from the annual reports of World Marrow Donor Association (WMDA). Public cord blood banks operate under strict international regulations. They must report to WMDA each year how many transplants they have released, and whether those releases stay in their native country or travel to another country.
In the graph below, we plot red bars with numbers on the right vertical axis displays cord blood transplants in India each year, either from public banks in India or shipped into India from other public banks. There were only two transplants per year in 2017 and 2018, and none since. Meanwhile, the blue line with numbers on the left vertical axis plots all cord blood transplants in the geographic region that the WMDA calls "Asia". In other Asian countries combined, there are over 1500 cord blood transplants per year.
Usually the answer is yes. However... some hospitals have signed exclusive contracts requiring their patients to use certain family cord blood banks, so it is best to check in advance.
Those hospitals that have exclusive partnerships with certain family cord blood banks will argue that it improves their level of care, because their staff train with and stock the collection kits for the banks they have approved. The hospital probably has a financial incentive too. However, for parents it means a lack of consumer choice.
The Parent's Guide to Cord Blood Foundation recommends that parents select a Family Bank whose laboratory has been inspected by an accreditation agency specific to cord blood banking: AABB or FACT. This provides a degree of quality assurance.
In some countries, national regulations hold Family Banks to the same standards as Public Banks, so an independent accreditation is not necessary. But in most countries the federal requirements for Family Banks are not as strict as Public Banks, and then a voluntary accreditation is desirable. For example, in the United States the FDA registers and inspects Family banks, but does not require them to have a Biologics License (BLA) like Public Banks.
Caveat: The process of registering with an accreditation agency and getting inspected can take a year, so it is understandable if a brand new lab does not have an accreditation yet.
You can preserve cord blood from twins in a family bank, but not in a public bank.
Public banks have a policy against accepting cord blood donations from twin births. One argument often heard is that the bank is afraid that the babies might be confused at birth. However obstetricians are trained to keep track of which baby is which in a multiple birth. The primary reason that public cord blood banks do not accept twin donations is that they have a size threshold for accepting donations. Twins tend to be smaller than single babies, hence they have less cord blood in the umbilical cord and the placenta, and in public banking it is crucial to get large donations. The chances are so low that both twins will have enough cord blood to meet the donation storage threshold that public banks just do not accept registrations from moms who are pregnant with twins.
Family banks have no problem accepting cord blood from twin births. Parents might have a problem paying for double cord blood banking, but almost every family cord blood bank offers special discounts for twin births. Some good news about banking from twins: the family bank Cryo-Cell ran a study which showed that their twins have enough cord blood for therapy.
Some parents ask if they should save the cord blood for both twins or only one? It is better biological insurance to save newborn stem cells from both twins. In the case of fraternal twins, the babies are distinct individuals with different DNA. In the case of identical twins, the babies have identical DNA. However, one identical twin may develop a genetic mutation later in life that causes a disease while the other twin does not. If that happens, then the healthy twin is the perfect matching stem cell donor, and there have been transplants where one identical twin donated cord blood to another.
Parents often complain about cord blood banking costs. This is not an industry where costs can be cut by running a turn-key operation. Each cord blood unit must be individually tested and processed by trained technicians working in a medical laboratory.
To explain why cord blood banking is so expensive in the United States, we wrote an article with the CEO of a public cord blood bank that lists the steps in cord blood banking and itemizes the cost of each one.
Another contributor to cord blood banking costs is the quality of the collection kit. Cheaper banks typically use flimsy collection kits. To insure the survival of newborn stem cells, the shipping container should be thermally insulated to maintain kit temperature during cord blood shipments.
A cord blood industry report by Parent’s Guide to Cord Blood Foundation found that, among developed nations, cord blood banking cost is only 2% of the annual income of those households likely to bank.
For those families who want to preserve newborn stem cells privately, our website lets parents compare cord blood bank prices in each country.
Donating your baby’s cord blood to a public bank is always free. The limitations of the public banking network in the United States are: they only collect donations at large birthing hospitals in ethnically diverse communities, the mother must pass a health screening, they prefer registration by 34 weeks of pregnancy, and they only save the largest cord blood collections. The potential reward of public donation is that your baby could Be The Match to save a life!
It is best to store cord blood in the country where the baby is born, provided a quality bank is available. If you currently live in one country and plan to store cord blood in your home country, make sure that your family bank has a shipping container that is well insulated and carries a temperature logger.
The important thing to know is that fresh cord blood that is traveling into the bank has a shelf life, whereas when the bank sends out cryogenically frozen cord blood to a clinic it does not expire.
After birth, the cord blood is shipped to the laboratory at room temperature. Every hour that it spends in transit, stem cells are gradually dying. Ideally the cord blood should arrive at the laboratory and be processed within 48 hours of birth. Sending the cord blood on a long airplane flight or a series of connecting flights is an additional risk for cell loss, unless the blood travels in the passenger compartment and is protected by a well-insulated shipping container.
By comparison, if a day should come where you need to use the cryopreserved stem cells, they will be shipped to the treatment center frozen and only thawed upon arrival. So on the release side of banking, cord blood stem cells can travel anywhere in the world with no loss of viability because they travel frozen.
Public cord blood banks throughout the world have adopted a time window of 48 hours as the maximum delay from birth to the initiation of lab processing. It would be a "best practice" if family banks also followed the 48 hour window.
Some data points:
- FACT accreditation standards require the 48 hour window for public donations but allow 72 hours for family banks.
- AABB accreditation standards do not specify a time window.
- The US FDA recommends the 48 hour window.
- The US state of NY Dept. of Health requires a 48 hour window.
In order to protect the internal kit temperature during cord blood shipments, Parent's Guide to Cord Blood Foundation recommends that parents select a family cord blood bank that has a well insulated transportation container, preferably with a temperature logger.
The standard protocol for transporting fresh cord blood is to keep it near room temperature within the range from 15 °C (59 °F) to 25 °C (77 °F). Priority shipping services may guarantee the arrival time, but they do not guarantee the temperature conditions during transit. Ambient temperature can get too hot or too cold while the package is sitting in the back of a truck, on a loading dock, or in the cargo hold of an airplane. This is why a well insulated container, preferably one that has been validated to IATA standards, is important.
In the United States, the post 9/11 security requirements of the Transportation Security Administration (TSA) require that specialty medical couriers can only offer cord blood shipping through cord blood banks that are registered with the TSA as a "Known Shipper". Before 9/11, specialty couriers could market their services directly to consumers, and in some countries this is still possible. Parents should check if their family bank offers specialty courier services before they sign a contract.
Accreditations are quality standards. We describe each one on our Accreditation Standards page.
Cord blood laboratories that are located in certain countries are required by federal law to follow high standards: these include GMP in Germany, HTA in the UK, Swissmedic in Switzerland and TGA in Australia.
In the United States, public cord blood banks are required to get a Biologics License from the FDA, but family cord blood banks are only required to register with the FDA and undergo surprise inspections.
There are two voluntary accreditation systems that have been developed specifically for cord blood banking and are based on inspections of the laboratory procedures: AABB and FACT. By comparison, ISO accreditation is not specific to cord blood banking. Parent’s Guide to Cord Blood Foundation recommends that parents choose a family bank that has AABB or FACT accreditation, if one is available in your country. In our 2015 Cord Blood Industry Report, we found that accredited cord blood banks are NOT more expensive on average that banks with no accreditation.
The three main components of cord blood, like any blood collection, can be separated by weight: the heaviest layer is the red blood cells (RBC), the lightest is the plasma (a clear white liquid), and in the middle is a pinkish layer called the "buffy coat" which contains the white blood cells (WBC), including stem cells. When banks process the cord blood, the final separated component that goes into storage is the buffy coat, even though only about 1% of the cells are actually stem cells. There is no procedure to separate out the stem cells alone.
The vast majority of blood processing methods rely on the different density of the three main blood components. They can be separated by sedimentation, or by centrifuge, or by a combination of the two techniques. The procedure can be performed manually by trained technicians or by automated machine.
Cryogenically frozen cord blood can be stored for decades and still be a viable source of stem cells for therapy. This conclusion is based on studies by Dr. Hal Broxmeyer, the man who invented cord blood storage in the 1980's. He contributed an article "How Long Can Cord Blood Be Stored?" for our newsletter of Sept. 2014.
Manual processing is when cord blood is handled in the laboratory by trained technicians. These technicians should be wearing gowns and gloves and handling the cord blood inside a laminar flow cabinet. Automated processing is when the cord blood collection bag goes into a functionally closed device like the Sepax or the AXP systems. In automated processing the laboratory technicians do not handle the cord blood during processing. When processing is completed by either manual or automated methods, a technician will always manually transfers the final storage bag to a controlled rate freezer
Advantages and Disadvantages of Automated Processing:
- Advantage: There are less opportunities for technician errors or the introduction of contaminants during processing.
- Automated processing may be a better approach in countries where it is hard to train and retain experienced technicians.
- Disadvantage: Automated processing is much more expensive. First, the laboratory has to purchase two devices, in case one breaks. Second, the devices use disposable kits which add significantly to the operating expenses.
- Automated processing makes sense in busy laboratories that handle a higher volume of cord blood business.
Advantages and Disadvantages of Manual Processing:
- Advantage: Manual processing is much less expensive.
- Manual processing makes sense in countries where highly skilled labor is easily available and affordable.
- Advantage: Manual processing is better for processing the smallest family collections because the procedures can be customized as needed.
These are all ways of counting cell types, and they tell you whether or not your cord blood collection has lots of stem cells and if they are healthy.
Stem cells happen to be Mono-Nuclear Cells or MNC: when you look at them under a microscope there is only one nucleus. Unfortunately, one of the most difficult aspects of stem cell biology is that you can't identify a stem cell just by looking at it. There are other types of blood cells which are also MNC, such as nucleated red blood cells. The only proof that a cell is a stem cell comes from how it behaves when it multiplies.
Scientists have worked for years to develop various chemical stains which have a high affinity for stem cells. The best known marker for blood-forming stem cells is that they test positive for CD34, a protein found on the surface of stem cells. But, CD34+ counts are not an accurate measure of stem cells: CD34+ results vary between labs, they can vary within a single lab, and only 1-2% of the MNC that have CD34+ are actually stem cells.
The Total Nucleated Cell count or TNC is the test most often reported as a measure of the cell count after cord blood processing. The main advantage of measuring TNC is that the count is highly reproducible within and among labs, so it can be used accurately throughout the blood banking community. Even better, the TNC count can be automated with the use of a device called a flow cytometer.
At present Colony Forming Units or CFU are considered to be the best measure of whether stem cells are "viable", or quite frankly alive. Unlike the TNC count, which includes both living and dead cells, the CFU test only reads living cells. The CFU test is run by taking a small portion of the cord blood and watching it under controlled conditions to see if stem cells divide and form colonies. This used to be a subjective measure, performed by a human with a microscope,, but in recent years it has been standardized with technology to image the cells and count colonies in the image. The only practical problem with this test is that it takes a week for colonies to grow. If the CFU test reads "zero", it means the cord blood does not contain living cells and is not worth banking
The earliest cord blood transplants were performed with whole cord blood. Thus, it is not absolutely necessary to process cord blood in order to save patient lives. There has never been a prospective randomized trial to compare transplant patient outcomes with whole cord blood versus processed cord blood.
Today almost all cord blood banks, both public and private, process cord blood before storage. The processing removes the plasma, so that the volume that goes into storage is reduced. The processing also removes the red blood cells, to avoid toxic effects from red cells that burst during freezing (see below). The final portion that is cryopreserved is called the "buffy coat"; it is the portion of the blood that holds both white blood cells and stem cells.
Researchers consider it important to remove red blood cells before preserving cord blood stem cells, and we have a fact sheet on red blood cell depletion. Red blood cells are removed because they tend to burst during freezing, which releases iron from the hemoglobin, and the iron can be toxic to patients. The alternate to removing the red cells before freezing is to wash any broken cells out of the cord blood unit upon thaw, but this washing step causes some loss of valuable cells.
- Does the bank collect the whole umbilical cord or only a segment?
- Does the bank freeze the umbilical cord intact or process it before storage?
- Does the bank store a tissue product (for example minced cord) or a cellular product (isolated cells from the tissue)?
- If the bank stores a tissue product, have they validated that upon thawing this tissue they can retrieve viable stem cells? This means not just testing for markers of stem cells but confirming there are cells that can grow in culture.
- If the bank stores isolated cells, what tests do they run to characterize the population of cells that go into storage?
- Does the bank culture the isolated cells to expand their numbers before storage?
- Does the bank offer any guarantees about the number of or quality of cord tissue stem cells that the bank will provide if the client requests them for therapy?
- Does the bank have AABB accreditation for tissue processing (a somatic cell activity)?
- Is the cord blood laboratory accredited by an agency that has specific standards for cord blood banks and conducts inspections? (ex: AABB, FACT)
- If you live in one of the US states that license cord blood banks (CA, IL, MD, NJ, NY) then you can only use a family bank that has your state license.
- Does the lab process cord blood around the clock, or only on selected shifts?
- What tests does the lab perform on maternal blood?
- What tests does the lab perform for infectious disease markers?
- What tests does the lab perform for contamination?
- Does the lab ever reject cord blood collections on the basis of the tests of maternal blood, infectious diseases, or contamination?
- Does the lab maintain a "quarantine tank" for the storage of blood while they are waiting for tests of infectious disease markers?
- What tests does the lab perform to measure the stem cell count of the processed cord blood and the stem cell viability?
- Does the bank inform parents, prior to storage, if the collection is too small for a transplant, and give them the option not to save it?
- Does the bank offer parents a refund if the cord blood collection has certain problems (contamination, low volume)? These refunds are typically only offered if the bank performed the collection as part of their service.
- What information will parents receive in the final report about their stored cord blood?
- How long has the company been banking cord blood?
- Who directs the day-to-day business of the company? Many cord blood banks have famous doctors on their Board of Directors; but they are not involved with the day-to-day operations.
- What is their inventory of stored cord blood units? Make sure to ask how many families banked cord blood with them. Many banks want to tell you how many "units" they have banked, but there could be multiple units per family (for cord blood, cord tissue, multiple storage containers)
- How many cord blood collections has the bank released for therapy?
- What instructional tools are provided in the kit for the physician and delivery staff?
- Will the family banking provider actively contact the labor and delivery staff for you?
- Does the hospital have designated staff that are trained to collect cord blood?
- What collection method does the bank use: gravity drip or blood draw?
- Is the collection blood bag sterile, both inside and out, so that it can be used in the operating room for a C-section?
- Does the bank provide the option of collecting additional stem cells from the tissue of the umbilical cord or the placenta?
- Are there any coupons currently available? Most banks are constantly running a "special limited time offer".
- Is the enrollment fee charged once per family, or for each birth?
- Is the first year of storage included in the processing fee?
- If there is an annual storage fee, is it guaranteed not to increase?
- Are there any discount programs? Some banks offer discounts to medical professionals or military personnel.
- Do parents have the option of a partial or full refund if they decide not to store the cord blood for any reason? For example, if the lab tests show contamination and the cord blood should not be saved, what happens? Full refunds are typically only offered in situations where the bank provided staff to perform the collection service.
- Should the family ever need the cord blood, will the bank charge to release it or to ship it?
- Is the cost of shipping included in the contract?
- Does the shipping company offer bed-side pick-up?
- Has the shipping container been tested for temperature stability?
- Does the shipping container include a temperature logger?
- On weekends, are the laboratory staff in-house or on-call?
- Does the bank guarantee to get the blood to the laboratory and processed within a certain time window?
- If the bank uses a medical courier, does the courier have possession of the cord blood throughout transit? (Sometimes the courier sub-contracts to another shipping company that is not a medical courier)
- What type of records do parents receive after storage?
- Does your contract state that the storage fee is fixed, or may it increase later?
- Does the bank reserve the right, in your contract, to change storage facilities?
- Does the bank operate their own storage facility, or is it provided by another laboratory?
- What type of accreditation does the storage facility carry? In most banks the cord blood is stored in the lab where it was processed, and the accreditation of the lab covers the storage conditions.
- What is the geographic location of the storage facility: Is it at risk for hurricanes, earthquakes, or other natural disasters?
- What type of back-up systems does the storage facility have in case of power failure?
- What type of security systems does the storage facility have?
In general sibling donors are better than unrelated donors for stem cell transplants. The exact comparison depends on the patient's diagnosis and the stage of disease.
The two important measures of patient outcome after a stem cell transplant are: long-term survival, and the amount of graft-versus-host disease (GvHD) that the patient suffers. Sibling donors trigger less GvHD, so that quality of life is better post-transplant. Also, sibling donors are available faster than searching for an unrelated donor, and patients have better survival when they go to transplant faster after diagnosis.
Some case by case studies: For many adult cancers the outcomes of transplants from siblings versus unrelated donors are comparable, although sibling donors have a slight edge. One large study was by Weisdorf et al. 2002, for over 2900 patients with CML leukemia. When correcting for all other factors, the survival with sibling donor vs unrelated donor was 68% vs. 61%. However, in pediatric transplants for hereditary disorders, sibling donors have a distinct advantage. The European Blood and Marrow Transplantation Group (EBMT) reported in 2011 that three year survival rates were 95% from a sibling donor vs. 61% from an unrelated donor.
The donor registry Be The Match has a section of their clinical website which reviews this topic here.
References:
Weisdorf, D.J. et al. Blood 2002; 99:1971-1977. doi:10.1182/blood.V99.6.1971
Bizzetto, R. et al. (EBMT) Haematologica 2011; 96(01):134-141 doi:10.3324/haematol.2010.027839
Childhood cancers are rare, and make up less than 1% of all cancers diagnosed each year1. It is even rarer for a child to require a stem cell transplant for cancer. In the US, the probability of a child having a stem cell transplant over the years from birth to age 20 is only 3 in 5000 or 0.06%2. This probability includes all 80+ of the diseases treated with cord blood.
Children that have a solid tumor have been treated with their own cord blood. This includes diagnoses such as neuroblastoma, medulloblastoma, and retinoblastoma. The very first case in the world where a child was given a transplant of her own cord blood happened in 1998 for a girl in Brazil who had neuroblastoma3.
Blood cancers such as the various forms of leukemia are the most common cancers in children. In the US, acute leukemia is one of the leading causes of death in children under 15 years of age4. Pediatric leukemia has been backtracked to birth5,6. Researchers have discovered that in most cases of pediatric leukemia the genetic mutation which triggered the leukemia started in utero, before the child was even born, and is present in the cord blood. In fact, researchers are now studying cord blood to learn which children are at risk for pediatric leukemia and how it can be prevented7,8.
Children should not be transplanted with their own cord blood if they have a pediatric blood cancer like leukemia, or a hereditary blood disorder like Thalassemia Major or Sickle Cell Anemia. All of these diseases have a genetic basis and they will be present in the child’s cord blood, so it is not safe to use that cord blood for transplant. Children with hematologic (blood) disease in need of transplant should receive stem cells from a donor. The ideal donor is a matched sibling donor.
References:
- American Cancer Society - Key statistics for childhood cancers
- Nietfeld JJ, Pasquini MC, Logan, BR, Verter, F, Horowitz MM. Lifetime Probabilities of Hematopoietic Stem Cell Transplantation in the U.S. BBMT 2008; 14(3)316–322.
- Ferreira E et al. 1999; Autologous cord blood transplantation. Bone Marrow Transplantation 24(9):1041.
- NIH Reporter. Backtracking Leukemia-Typical Somatic Alterations in Cord Blood at Single-cell Resolution. Project Number 1R01CA262012-01
- Ford AM, Bennett CA, Price CM, Bruin MCA, Van Wering ER, Greaves M. Fetal origins of the TEL-AML1 fusion gene in identical twins with leukemia. PNAS 1998; 95(8):4584-4588.
- The Institute of Cancer Research (ICR). Pioneering scientist Mel Greaves is knighted after research to unveil cause of childhood leukaemia. News archive Published 2018-12-28
- Marcotte EL, Spector LG, Mendes-de-Almeida DP, Nelson HH. The Prenatal Origin of Childhood Leukemia: Potential Applications for Epidemiology and Newborn Screening. Frontiers Pediatrics 2021; 9:639479.
- Spector L. Studying Cord Blood to Prevent Childhood Leukemia. Parent's Guide to Cord Blood Foundation News Published 2022-07
The crucial thing is not the volume of the cord blood collection, but the number of stem cells it contains. Transplant doctors develop recommendations based on the Total Nucleated Cell count, or TNC, because it is the easiest measure to reproduce between different labs.
When treating cancer, the transplant dose should be at least 25 million TNC per kilogram of patient body weight (1 kilogram equals 2.2 pounds). The average cord blood collection holds 8.6 million TNC per mL. Thus, the optimal transplant dose requires harvesting:
1.3 mL of cord blood for every pound of patient weight, -or-
2.9 mL of cord blood for every kg of patient weight
References:
Reed, W et al., Blood 2003;101(1):351 doi:10.1182/blood-2002-02-0394
Barker, JN et al., Blood 2005;105:1343-1347 doi:10.1182/blood-2004-07-2717
Eapen, M et al. Lancet 2007;369:1947-54 doi:10.1016/S0140-6736(07)60915-5
Rocha & Gluckman Brit. J. Haematology 2009;147(2):262-274 doi:10.1111/j.1365-2141.2009.07883.x
Yes, stem cell transplants with cord blood have been used to cure both children and adults with leukemia since the early 1990's. To date, there have been over 35,000 cord blood transplants world-wide, and most of them were for leukemias and other blood disorders (Ballen Verter Kurtzberg 2015). A study published in the New England Journal of Medicine (NEJM) in Sept 2016 compared cord blood transplants versus bone marrow transplants for leukemia patients. The two groups had comparable survival post-transplant, but the cord blood patients tended to live longer and most importantly the cord blood patients were less likely to relapse.
The important caveat is that children with leukemia or another blood disorder must receive a cord blood transplant from a donor, NOT their own cord blood. It turns out that when children and even adolescents develop leukemia, they were born with the genetic defect that triggered the leukemia... hence it is not safe to give them a transplant with their own cord blood because it probably carries the mutation for leukemia.
References:
Backtracking leukemia to birth: Gale KB et al. 1997; Proc Natl Acad Sci USA. 94(25):13950-4. PMID:9391133
Backtracking leukemia to birth: Janet D. Rowley 1998; Nature Medicine 4:150-1 PMID:9461182
Ballen KK, Verter F, Kurtzberg J 2015; Bone Marrow Transplantation 50(10):1271-8. doi:10.1038/bmt.2015.124
HealthDay article describing study in Sept 2016 NEJM: Cord Blood Transplants Show Promise in Leukemia Treatment
Filippo Milano, et al. 2016; NEJM 375:944-953. DOI:10.1056/NEJMoa1602074
The term "HLA" is short for Human Leukocyte Antigens, and these are proteins in the immune system that determine whether a patient will react against a donor transplant. A very good basic tutorial about HLA types is on the Stanford Website, and the donor registry Be The Match explains the role of HLA Typing and Matching in stem cell transplants on their clinical website.
Briefly, there are 6 HLA types that are important for stem cell transplants: in a bone marrow transplant the patient and donor must match at all 6 (100% match), whereas a cord blood transplant is just as effective at curing patients with only a 4 out of 6 match (67% match) between donor and patient. This is the reason that cord blood donations are so important to help patients who come from minority or mixed racial backgrounds.
The HLA type of cord blood is always measured by public banks, and then the type is listed on a registry that can be searched by patients seeking a transplant. Family banks typically do not measure the HLA type at the time of banking, because it is an expensive lab test and and can always be checked later from a testing segment of the stored cells.
The odds of using your baby's cord blood are the same as the probability that your baby or a close family member will have a disease that can be treated with cord blood.
Family cord blood banks tell parents that there are 80 diseases for which stem cell transplants are a standard treatment. That is a true statement, but it can be misleading. Most of those 80 diseases are rare among children. In the United States, the net probability that a child will need any type of stem cell transplant by age 20 is 3 in 5,000 or .06%. So the odds of use for transplant of a child are only 3 in 5000 for all of the 80 diseases combined!
When does cord blood stored in family banks have significant odds of use?
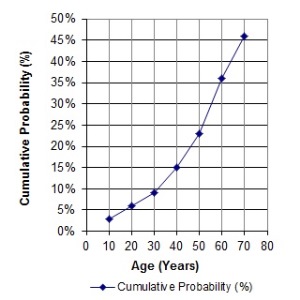 Family members: The graph on the left illustrates that, as people get older, rates of cancer increase, and the cumulative probability of having a stem cell transplant increases. In the United States, 1 in 217 people, or .46%, will have a stem cell transplant (not just need one, but have one) by age 70. Hence the cord blood that parents store from their baby might be of help to an immediate family member years from now. The cord blood is most likely to match first degree relatives: full siblings and parents.
Family members: The graph on the left illustrates that, as people get older, rates of cancer increase, and the cumulative probability of having a stem cell transplant increases. In the United States, 1 in 217 people, or .46%, will have a stem cell transplant (not just need one, but have one) by age 70. Hence the cord blood that parents store from their baby might be of help to an immediate family member years from now. The cord blood is most likely to match first degree relatives: full siblings and parents.
Inherited disorders: The odds of use quoted for the average person in the United States do not apply to some families and do not apply at all in other countries.
For example, some parents want cord blood banking because they have many relatives with an auto-immune disorder like Multiple Sclerosis, and they know that stem cell transplants show promise for auto-immune diseases.
In Asian countries where the inherited blood disorder Thalassemia is prevalent, family cord blood banks are filling a public health need. Families can bank cord blood from a healthy baby to provide a sibling cord blood transplant to an older child with thalassemia. In Thailand some families are using assisted reproduction technology to conceive a matched savior sibling for an older child with Thalassemia.
In Africa, cord blood banks could fill a public health need by providing cord blood transplants for sickle cell disease and by storing stem cells that have a genetic mutation that can combat HIV.
Regenerative medicine: Parents are most likely to need their baby's cord blood to treat neurological disorders that are diagnosed in the first few years of life. These include autism, cerebral palsy, hypoxic-ischemic encephalopathy (HIE), apraxia, ataxia, hydrocephalus, in-utero stoke, traumatic brain injury, and similar conditions.
No one wants to imagine that their child might be born with a brain injury, but the reality is that cerebral palsy (CP) occurs in 2 of 1,000 full term births, and among premature births it is 10 times more common at 2 in 100 premies or 2% have cerebral palsy. Another relatively prevalent condition that may benefit from trials of cord blood therapy is autism spectrum disorders (ASD), which impact 1 in 44 US children as of 2018. As a public service, we have made available for download a spreadsheet of all clinical trials treating CP or ASD with cell therapy that were registered worldwide from 2017 to 2021.
Please see our page Cord Blood Odds of Use, which gives numbers and has many references.
Over the past decade, there have been over three dozen clinical trials treating cerebral palsy with stem cells. Several of these trials had outcomes, published in peer-reviewed medical journals, which showed that cerebral palsy patients who received stem cells had a significant improvement compared to patients in control groups. It is hard to compare one trial against another, because a variety of stem cell types have been tested, and the trials differed in how and when they measured patient responses. Nonetheless, a 2023 meta analysis of multiple trials showed that there is statistically significant benefit from cord blood therapy for cerebral palsy.
Despite these successful reports, no one understands the “mechanism of action” by which the stem cells provide a benefit. Both cord blood stem cells and cord tissue MSC are known to suppress inflammation. Researchers at Duke have argued that a trace component of the cells in cord blood may enable re-myelination of neurons in the brain. It is presumed that stem cell therapy probably also benefits other conditions that are similar to cerebral palsy, such as hypoxic ischemic encephalopathy. Bear in mind these benefits were proven for groups of patients and individual outcomes will vary. While some children have had dramatic improvements, for example Adriana or Asia, others have not improved at all. There are many open questions about stem cell therapy for cerebral palsy that have not yet been answered by the existing research. It is not clear what type of stem cells are the “best” for this therapy, and there is also some debate over the optimum method of administering the stem cells (intravenous drip versus intrathecal injection).
On the basis of the available evidence, Duke University has received permission from the FDA to offer expanded access to cord blood therapy for cerebral palsy and other pediatric brain injuries. Any family that has a child with an acquired (not hereditary) brain injury, and has that child’s cord blood or a sibling’s cord blood in storage, is eligible for this therapy.
An important caveat for parents is that while stem cell therapy may improve a child’s cerebral palsy symptoms, it is not a cure all and does not replace other supports, such as physical and occupational therapy, tutoring, etc. Please remember that the website of Parent’s Guide to Cord Blood Foundation is not a substitute for medical advice from a physician.
This topic is so somplex that it became too long for a FAQ post. We created a special page to cover Everything parents should know about stem cell therapy for autism. Most important, it explains the pros and cons of therapy with cord blood MNC versus cord tissue MSC.
Indefinitely. From an economic perspective, it does not make sense to invest in the up-front processing fee and pay for years of annual storage, and then throw out the investment. That would be like buying life insurance and then cancelling it because you have not died yet. Especially given that the probability of some one in the immediate family needing a transplant increases with age. Even if the cord blood collection was small, and the child becomes too large to use it for a transplant, it could still be enough cells for a regenerative medicine therapy. Stem cells which have been cryogenically preserved remain viable for decades. See How long can cord blood be stored?
References:
Mazur, P. Science 1970; 168(3934):939-949
Nietfeld, J.J. et al. BBMT 2008; 14:316-322
Broxmeyer, H.E. Stem Cells Translational Medicine. 2023; 12(S1)
All the reasons that you banked for the first child are still valid for additional children.
1. If you want the baby to have the option of using his/her own cells, then you need to bank them.
2. If you are banking as a form of "biological insurance" for siblings, then the ability to use cord blood from one child for another depends on whether they have matching HLA type. Two full siblings have a 25% chance of being a perfect match, a 50% chance of being a half match, and a 25% chance of not matching at all. The more siblings with banked cord blood, the more chance that they cover each other for possible transplants or other therapies for which sibling stem cells are an option.
Reference:
Odds of sibling match are based on haplotype inheritence: that the child will receive 3 HLA types as a group from each parent.
In general, NO. The obligation of family banks is to store the cord blood. The contract between the parents and the bank does not cover the costs of therapy. In some countries the costs of a cord blood transplant are covered by the national health service. If you live in a country where these treatments are not free or would like to have the option to have them done abroad, we recommend searching for a bank that has an insurance partner providing cord blood insurance, or perhaps you can purchase a separate insurance policy for this coverage.
Some family banks provide forms of insurance that either guarantee their banked samples or help their client families pay for therapy using cord blood.
- Engraftment insurance is a policy that will pay the family a lump sum if they try to use the cord blood for therapy and the stem cells fail to grow, or engraft.
- Some family banks offer families a payment if a child gets sick with an illness that requires a cord blood transplant.
- Community banks usually offer families a payment to help them pay for the cost of therapy when they make a withdrawal from the bank.
When parents want to treat their child with cord blood, the out-of-pocket cost can vary enormously, depending on the diagnosis being treated and the institution where they get the treatment.
Generally, if your child has one of the 80+ standard diagnoses that are treated by stem cell transplant, the cost of treatment should be covered by your health insurance. You would either by covered by your national healthcare program (for example in European countries) or by your personal health insurance (for example in the United States). If you are enrolled in a "Community" cord blood bank (for example in India) then your customer service package includes a lump sum payment for therapy. When cord blood transplants are considered one of the standard care options for the diagnosis, you can usually rely on your healthcare provider for a referral to a treatment program. In some hospitals, doctors will not consider cord blood transplants because they are not trained to perform them. If parents have doubts about why their doctor is steering them to one therapy choice over another, it is always a good idea to get a second opinion from a specialty center.
If your child is diagnosed with a condition where cord blood therapy is not yet a standard of care (such as cerebral palsy and other complications of prematurity) then finding affordable treatment gets more difficult. If you enroll in a clinical trial that is being conducted by a research center, then the cost of the therapy will be covered by the trial. However, parents still have to pay for travel and lodging costs associated with the trial, unless they can find a charitable program to help with these expenses (for example, Ronald McDonald House).
If parents want to try an experimental program at a commercial clinic (for example cord blood or MSC for autism), they can expect to pay thousands of dollars out of pocket. Regardless of whether the clinic has registered the treatment as a "clinical trial", if the clinic is commercial in nature and not a research institution then the parents have to cover all the costs. There are a number of support groups (for example on Facebook) where parents compare notes on the cost of various commercial clinics and how many cells you get for the price.
The term "cord tissue" refers to the rest of the umbilical cord, other than the blood. A normal umbilical cord contains two arteries, one vein, an outer skin, and it is filled with a gelatinous material called Wharton’s Jelly. Umbilical cords can vary widely in length, but the average cord is about 61 cm (24 inches) long and weighs 40 gm.
Reference:
Percy Malpas 1964; British Medical Journal 1:673–674.
Like cord blood, the umbilical cord tissue is a rich source of progenitor cells. However, they are a different population of cells from the ones in cord blood. While most of the stem cells in cord blood are blood-forming or hematopoietic stem cells (HSC), most of the cells in cord tissue are mesenchymal stromal cells (MSC). The MSC are not distributed uniformly in the cord, but are mostly clustered around the walls of the blood vessels. The typical umbilical cord is estimated to hold 11 million MSC per gram of tissue.
While MSC can be found in many parts of the adult human body, MSC from the umbilical cord or UC-MSC are the most commonly used in clinical trials of regenerative medicine. It is well established that they can modulate the immune system and suppress inflammation.
Reference:
Schugar RC et al. 2009; Journal of Biomedicine and Biotechnology 2009:789526 (open access)
Cord blood banking has become mostly standardized over the past 25 years, but cord tissue banking is still an evolving field and industry standards are still being developed. For example, in cord blood banking the standard method of processing is to separate the component of the blood that holds stem cells and cryogenically freeze the isolated cells.
Over half of family cord blood banks also offer cord tissue storage, but their methods of processing the tissue differ widely. Some banks merely freeze the cord intact with no attempt to process it. The majority of banks that offer cord tissue banking do process the cord, but the final biological product that they store may be either a tissue product (for example very small pieces of cord), or a cellular product of isolated cells (the same as cord blood storage), or both. The Parent’s Guide to Cord Blood Foundation is working to develop educational materials to help both parents and professionals navigate this rapidly evolving field.
References:
- Silva Couto P. Storage of Mesenchymal Stem/Stromal cells in family stem cell banks: What do they offer? Parent's Guide to Cord Blood Newsletter May 2014
- Parent's Guide to Cord Blood Foundation 2016; poster #1606 at International Cord Blood Symposium, Transfusion doi:10.1111/trf.13686
- Bapat A, Silva Couto P, Verter, F. Buyer Beware: Parents should demand quality testing of umbilical cord tissue storage. PGCB Newsletter Jan 2018
The tissue of the umbilical cord is rich in the primitive mesenchymal stromal cells (MSC). These cells can differentiate to bone, cartilage, and fat. More importantly, MSC send signals that modulate the patient's immune system and suppress inflammation. Infusions and injections of MSC are frequently used to treat orthopedic conditions, chronic pain, and auto-immune disorders. Clinical trials with MSC from cord tissue are available for many different disorders.
Reference:Arnold Caplan PhD & Diego Correa, MD PhD. THE MSC: AN INJURY DRUGSTORE Cell Stem Cell. 2011; 9(1):11–15.
Just like cord blood banking for the family, cord tissue banking is a form of biological insurance, where parents bank their baby’s stem cells for future therapies.
The two main benefits of banking both cord blood and cord tissue are: (1) to have more stem cells from the same child and (2) to have different types of cells.
However, parents should not feel obligated to spend extra money to bank cord tissue. The reason is that, unlike cord blood, therapies with cord tissue do not require matching the donor and patient. Should you or your child ever need a treatment with cells from cord tissue, it will be easier and less expensive to purchase an off-the-shelf cell therapy product, rather than try to make a custom therapy from you child's cells. There will not be any advantage to having your own cells, since the cells do not need to be matched.
The tissue of the umbilical cord and the placenta are rich sources of mesenchymal stromal cells (MSC). Currently MSC are the most popular form of cells that are being used in regenerative medicine and appear in many papers of published research. MSC show great promise for treating a wide variety of auto-immune disorders and treating injuries to muscle or bone (heart disease, sports injuries). The MSC from birth sources, such as cord tissue and the placenta, grow faster than MSC from adult donors and have never been exposed to disease.
References:
Perinatal Stem Cells 2nd edition 2013; book published by Wiley
Perinatal Stem Cells 3rd edition 2018; book published by Elsevier
There are big differences between cord blood therapy versus therapy with Mesenchymal Stromal Cells (MSC). Here is a quick comparison
Property | Cord Blood | MSC |
‘stem’ cells can become: | blood and immune system | muscle, bone, fat |
Source | Blood | Tissues: umbilical cord, placenta, bone marrow, adipose (fat), etc. |
Requires HLA match ? | Yes | No |
Requires chemotherapy? | Yes before a transplant; | Never |
Administered | IV infusion, Intrathecal injection | IV infusion, Intrathecal injection, |
Unlike cord blood, MSC do not contain immune system cells. There is no chance of the donor and patient rejecting each other and triggering "graft versus host" disease. This makes MSC very versatile - a patient does not have to find a sibling or matched donor, a patient can receive MSC from any unrelated donor. Medical researchers agree that, when properly prepared, it is safe to use MSC therapy products that are “off-the-shelf”.
During the past decade, over a thousand clinical trials have given a variety of MSC from different sources to over 55 thousand patients. Although MSC have been tried for many conditions, in most cases the correct dose of MSC still has not been established. There are also questions about the relative benefits of big doses versus repeated smaller doses.
The rate of complications from MSC treatments in clinical trials has been very low and the complications were mostly mild. But commercial clinics may not be as safe as clinical trials, and the regulations on commercial clinics varies between countries (we have articles about Mexico and UAE). The FDA has issued “warning letters” to a number of commercial clinics in the United States that supposedly give injections of MSC from birth tissues. Oftentimes their product does not actually contain live stem cells, or even worse they may be contaminated with bacteria. Consumers should check the credentials and quality control practices of any clinic and their suppliers before purchasing MSC therapy.
References:
- Ankrum JA, Ong JF, and Karp JM. Nature Biotechnology 2014; 32:252–260. Mesenchymal stem cells: immune evasive, not immune privileged
- FDA: For Consumers - FDA Warns About Stem Cell Therapies
Therapies with mesenchymal stromal cells (MSC) from all sources are still under research, so the optimum dose has not been established for any diagnosis.
As of August 2020, only two papers have been published that summarized the MSC cell doses used in clinical trials. The first paper, by Couto et al. 2019, looked at the first decade of clinical trials with MSC from umbilical cord tissue (UC-MSC), over the years 2007-2017, and collected trials from everywhere in the world. The trials were divided between those that gave local injections of UC-MSC versus systemic administration into the blood stream. Their figure 4 showing number of trials versus total cell dose is repeated below. Please note that there are breaks in the dose axis to accommodate the very wide range of cell doses.
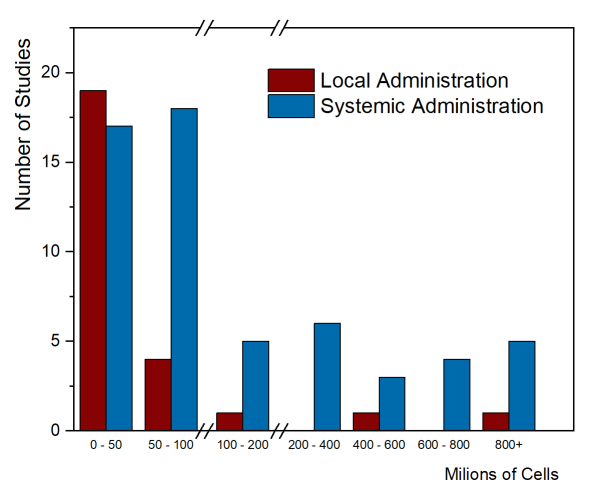
Clinical applications that employ systemic administration tend to have higher cell doses. It is not possible to give large doses locally, such as injecting a single arthritic joint or injecting the spinal cord. The few trials that achieved high total cell dose with local administration were trials that gave multiple doses. In this graph, the median total dose of the studies with local administration was 18.75 million cells, while the median total dose of the systemic administration studies was 80 million cells.
A second paper published by Kabat et al. 2020 did the same type of analysis with a different data set of clinical trials. They looked at clinical trials with any type of MSC (from bone marrow, fat tissue, umbilical cord tissue, etc.), over the years 2004-2018, but only the trials registered in the United States. Within their data, they divided the route of administration into six different groups. They looked at route of administration versus trial phase, cell dose, and patient diagnosis (see their figures 5A – 5C). The most common method of delivery was intravenous (systemic) administration and the median dose by that route was 100 million cells.
References
- Couto PS, Shatirishvili G, Bersenev A, and Verter F. 2019; Regenerative Medicine 14(4):309-319. First decade of clinical trials and published studies with mesenchymal stromal cells from from umbilical cord tissue
- Kabat M, Bobkov I, Kumar S, Grumet M. 2020; Stem Cells Translational Medicine 9(1):17-27. Trends in mesenchymal stem cell clinical trials 2004‐2018: Is efficacy optimal in a narrow dose range?
All cord blood banks in the United States are required by law to be registered with the FDA. The FDA conducts surprise inspections of facilities on this list. So yes, family cord blood banks are inspected by the FDA, and they never know when it will happen, so they must adhere to standards at all times.
Within the United States, some states have licensing requirements for cord blood banks. These requirements apply to any cord blood bank that operates within the state, accepts collections from the state, or distributes cord blood units to the state.
If you are a parent who lives in a state with a licensing requirement, then only those cord blood banks that are licensed by your state can legally do business with you. Licenses are required for the states of California, Illinois, Maryland, New Jersey, and New York.
The states California and New York have the strictest licensing standards. These states have inspectors that visit cord blood banks to confirm compliance with the licensing requirements.
The short answer is NO, the typical consumer does not have the technical knowledge to properly evaluate companies that provide biobanking services.
Newborn stem cell banking is a healthcare service and you should pick a bank based on the healthcare quality metrics that we explain on this website: whether the laboratory is accredited for cord blood or cord tissue banking, has experience providing therapies to patients, and is financially stable.
Parents should be wary that most “consumer ratings” type websites are rigged to give the highest ratings to companies that pay the most for advertising. Another problem is that the reviews on consumer websites may not have been written by real customers.
Parent’s Guide to Cord Blood Foundation does not endorse banks. We do favor our donors by placing their descriptions at the top of the list of banks in their country. However we do not allow banks to make any claims in their descriptions unless they can be substantiated.
These rumors are totally false. In fact, our 2015 Cord Blood Industry Report found that the majority of family cord blood banks in the United States and Canada are processing cord blood manually with hespan. Hespan or HES is a brand name for the chemical hydroxyethyl starch. It is commonly used in most laboratories that handle blood.
The source of the rumors about hespan is the following: Up until recently, it was a standard procedure in emergency rooms to give a large infusion of hespan to patients who were going into shock from loss of blood pressure. The idea was to briefly replace their blood volume with hespan while the ER doctors were rushing to fix whatever problem had caused a rapid loss of blood pressure, and while waiting for a matching transfusion from the nearest blood bank. However, retrospective studies have recently found that patients who survived this experience were likely to develop kidney failure later. Hence, doctors now realize that infusing large volumes of hespan intravenously is not safe. However, the use of small volumes of hespan in cord blood processing is still perfectly safe.
Reference:
Zarychanski R. et al. 2013; JAMA 309(7):678-88. doi:10.1001/jama.2013.430.
This claim is mis-leading. The exact wording of their advertising is "up to twice as many" stem cells compared to other cord blood banks. Quite frankly, if it really were true that their processing method is so efficient, then the other cord blood laboratories would have rushed to copy it.
The bank in question is a marketing company that does not operate their own laboratory; they sub-contract laboratory services to an independent company. Moreover, they have switched laboratories multiple times. It is very hard to believe that their methods are twice as good as the proprietary services that established banks have developed with in-house research.