Вы здесь
MSC Proven Effective for Cerebral Palsy
 The cord blood community has been pleased by the publication of papers which demonstrate that cord blood stem cells have efficacy as a therapy for cerebral palsy, when tested against controls in double-blind trials1-3.
The cord blood community has been pleased by the publication of papers which demonstrate that cord blood stem cells have efficacy as a therapy for cerebral palsy, when tested against controls in double-blind trials1-3.
However it is important to note that Mesenchymal Stem/Stromal Cells, or MSC hereafter, have also been proven effective for cerebral palsy in studies against controls4,5. From the perspective of cord blood bankers, this is not a “competing” protocol, because MSC can be sourced from the baby’s own umbilical cord and/or placenta. Since the majority of private cord blood banks now provide storage of perinatal tissues6, the use of MSCs as therapy for cerebral palsy is also a business opportunity for this industry.
In this article we highlight results from two studies which both used MSC to treat cerebral palsy and both had control groups. These two studies both measured patient outcomes on the GMFM-88 scale7, and hence they are directly comparable. When comparing cell therapies for cerebral palsy, an important caveat is that the GMFM scores of children improve over time without intervention, so that only improvements versus controls are meaningful8.
The first paper was published4 in 2017 by the group of Dr. Yihua An at the Dept. of Cell Transplantation in the General Hospital of Chinese People’s Armed Police Forces in Beijing China. The study group consisted of 35 children (54% male) with a mean age of 4.1 years (range 0.8-12.5) who were all diagnosed with spastic cerebral palsy. The patients received autologous MSC that had been harvested from their bone marrow (BM-MSC) and was cultured to passage 4-6. The MSC were delivered by intrathecal injection with a dose of 1 million cells per kg of body weight. This procedure was repeated four times at intervals of 3-4 days. At 12 months post-treatment, the GMFM-88 scores of the patients had risen from 95.21 +/- 32.69 to 127.03 +/- 35.80, whereas the control group scores had gone from 95.26 +/- 29.19 to 102.51 +/- 28.30. In other words, the patients had a net improvement of 24.52 against controls on the GMFM-88 scale, and this was statistically significant at the P<0.01 level.
The second paper was published5 in 2018 by the group Dr. Haixia Lu at the Inst. of Neurobiology in the School of Basic Medical Sciences in Xi’an China. The study group consisted of 27 children (81% male) with a mean age of 7.3 years (range 3-12) who were all diagnosed with cerebral palsy. The patients received allogeneic MSC that had been harvested from cord tissue (UC-MSC) by the company Beike Biotechnology, and the researchers cultured them to passage 4. The MSC were delivered by intravenous infusion with a fixed dose of 50 million cells without immune suppression. This procedure was repeated four times at intervals of 1 week, and then the set of 4 infusions was repeated 3 months later, so that each patient received 8 doses with a net cell count of 400 million. The baseline GMFM-88 scores were 84.99 +/- 0.85 for the patients and 85.03 +/- 0.76 for the control group. At 24 months post-treatment, the GMFM-88 scores of the patients had risen by 12.66 +/- 0.66, whereas the control group scores increased by 4.81 +/- 0.39, or in other words the patients had a net improvement of 7.85 on the GMFM-88 scale, and this was statistically significant at the P<0.05 level.
The sizes of the cell doses in these studies deserve a closer examination. In the intravenous study the dose was fixed at 50 million cells repeated 8 times, so that every patient received a net dose of 400 million MSC. The intrathecal dose was 1 million cells per kg repeated 4 times. The patient weights are not provided in the paper, but based on child growth standards from the World Health Organization9, we know that a boy with a mean age of 4.1 years has a mean weight of 16.5 kg. This allows us to estimate that the mean net dose in the intrathecal study was 66 million MSC.
Based on this preliminary comparison of two separate clinical studies, it appears that the intrathecal route of administration is more effective for the treatment of cerebral palsy. However, these are two different studies that, in addition to using different delivery routes, also employed different MSC sources, have different mean patient age, different rehabilitation for the control patients, etc. It is a provocative comparison but not conclusive.
Going forward we need more research that compares the impact of delivery route on the efficacy of cell therapy. A paper published in 2015 found that while the intracerebroventricular (IC) administration delivered more MSC to the brain than the intravenous (IV) route, nonetheless IC and IV methods of delivery had equal therapeutic efficacy10,11. However, that study treated newborn rats, so further research is needed in humans.
A human clinical trial NCT03414697 that launched in January 2018 plans to compare routes of UC-MSC delivery in children with cerebral palsy. The tested routes will be intravenous, intrathecal, and intranasal delivery. This study will be performed by the group of Dr. Jing Liu at the First Affiliated Hospital of Dalian Medical University in Dalian China, and outcomes will be measured on the GMFM-88 scale.
For now, we believe that clinicians should keep an open mind about what is the best type of cell therapy and the best method of delivery for the treatment of neurological disorders such as cerebral palsy and autism.
References
- Min K, Song J, Kang JY, Ko J, Ryu JS, Kang MS, Jang SJ, Kim SH, Oh D, Kim MK, Kim SS & Kim M. Umbilical Cord Blood Therapy Potentiated with Erythropoietin for Children with Cerebral Palsy: A Double-blind, Randomized, Placebo Controlled Trial. 2013; Stem Cells, 31(3):581-591.
- Kang M, Min K, Jang J, Kim SC, Kang MS, Jang SJ, Lee JY, Kim SH, Kim MK, An SSA, & Kim, M. Involvement of Immune Responses in the Efficacy of Cord Blood Cell Therapy for Cerebral Palsy. 2015; Stem cells and development, 24(19):2259-2268.
- Sun JM, Song AW, Case LE, Mikati MA, Gustafson KE, Simmons R, Goldstein R, Petry J, McLaughlin C, Waters‐Pick B, Chen LW, Wease S, Blackwell B, Worley G, Troy J, & Kurtzberg J. Effect of Autologous Cord Blood Infusion on Motor Function and Brain Connectivity in Young Children with Cerebral Palsy: A Randomized, Placebo‐Controlled Trial. 2017; Stem Cells Translational Medicine, 6(12):2071-2078
- Liu X, Fu X, Dai G, Wang X, Zhang Z, Cheng H, Zheng P, & An Y. Comparative analysis of curative effect of bone marrow mesenchymal stem cell and bone marrow mononuclear cell transplantation for spastic cerebral palsy. 2017; Journal of Translational Medicine, 15:48
- Huang L, Zhang C, Gu J, Wu W, Shen Z, Zhou X, & Lu H. A Randomized, Placebo-Controlled Trial of Human Umbilical Cord Blood Mesenchymal Stem Cell Infusion for Children With Cerebral Palsy. 2018; Cell Transplantation 27(2):325-334
- Verter F. and Couto PS. Cord Blood Industry Report. 2015; Parent’s Guide to Cord Blood Foundation Nov. 2015 newsletter
- Rosenbaum PL, Walter SD, Hanna SE, et al. Prognosis for gross motor function in cerebral palsy: creation of motor development curves. JAMA. 2002;288:1357–1363
- Verter F. Cerebral Palsy Cell Therapy Comparison. 2018; Parent’s Guide to Cord Blood Foundation April 2018 newsletter
- WHO Child Growth Standards, table for boys ages 0 – 5 years
- Ahn SY, Change YS, Sung DK et al. Optimal Route for Mesenchymal Stem Cells Transplantation after Severe Intraventricular Hemorrhage in Newborn Rats. 2015; PLoS ONE 10(7):e0132919
- Cohen-Pfeffer JL, Gururangan S, Lester T et al. Intracerebroventricular Delivery as a Safe, Long-Term Route of Drug Administration 2017; Pediatric Neurology 67:23-35



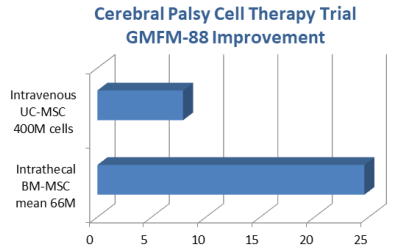 The average patient improvement in the study with intrathecal cell delivery was three times as large as the study with intravenous route of administration, despite employing a net cell dose that was six times lower.
The average patient improvement in the study with intrathecal cell delivery was three times as large as the study with intravenous route of administration, despite employing a net cell dose that was six times lower.