Вы здесь
Developing new treatments for Traumatic Brain Injury
Traumatic brain injury (TBI) is considered to be a major cause of disability and death worldwide, especially in children (as a result of falls and playground injuries), soldiers (from blasts and accidents), and the elderly (falls and stroke). The incidence of TBI is 235 per 100,000, with a worldwide mortality of about 1.5 million per year, and in the USA more than 5 million people are coping with disabilities from TBI at a cost of $60 billion a year.
Symptoms of TBI are dependent on the type of TBI (diffuse or focal), the part of the brain that is affected, and the injury's severity. Briefly, the symptoms can include loss of consciousness, headache, vomiting, nausea, dizziness, balancing difficulties, blurred vision, ringing in the ears, bad taste in the mouth, fatigue or lethargy, and changes in sleep patterns. Cognitive and emotional symptoms include behavioral or mood changes, confusion, and trouble with memory, concentration, attention, or thinking.
In addition to the damage caused at the moment of and in the days following the injury, brain trauma causes delayed secondary events, including: initiation of an inflammatory cascade, blood-brain barrier disruption, degradation of lipids in the brain by oxidation, and degeneration of neurons. These series of events lead to the development of various neurological deficits.
The best treatment for TBI is prevention. Once brain injury does happen, the treatment depends on the recovery stage of the patient. In the acute stage, the primary aim is to stabilize the patient and focus on preventing further injury. Rehabilitation is then the main treatment for the sub-acute and chronic stages of recovery.
Currently, there is no actual designated treatment for TBI, or for preventing the progression of secondary degeneration from TBI. Despite promising pre-clinical studies, over the past decade, 30 phase 3 clinical trials have failed to show significant results at reducing secondary injury following TBI. Halting the secondary degeneration requires an intervention that simultaneously targets multiple factors which contribute to the progress of the neuro-degradation in the brain.
In recent years, stem cells have been embraced as a therapy for TBI. Stem cells can halt brain damage and promote healing by migrating to the site of injury where they reduce inflammation and have a tissue-protective effect. Pharmacological pre-clinical studies have demonstrated that treatment with autologous stem cells administered immediately after trauma may aid in reducing the immediate cognitive defects of TBI (1). Clinical studies in patients with TBI have demonstrated that neurologic function was improved at 6 months after cell therapy (2). Currently, there is an ongoing phase 1 clinical trial where children with TBI receive stem cells from cord blood.
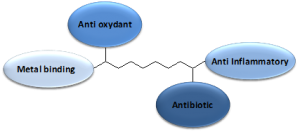
At MediCortex USA Ltd., we are developing an alternative treatment that simultaneously targets multiple processes in TBI. The company has designed a family of chemically verified small molecules, called New Chemical Entities (NCEs) that aim to decrease levels of circulating toxic metal ions in the brain, as well as minimize oxidation by free radicals. The NCEs, each with the ability to cross the brain-blood-barrier, have at least two more active sites that exert neuro-protective functions, such as free metal ion binding, anti-oxidation, anti-inflammation, and/or anti-bacterial (see the diagram). At present the molecules are being tested in pharmacological pre-clinical studies.
If successful, small molecules have many advantages over stem cells as emergency intervention for TBI patients. Only TBI patients whose parents stored their cord blood and cord tissue would have their own stem cells readily available for treatment. Otherwise, TBI patients pursuing stem cell therapy must undergo an invasive stem cell harvest or obtain donor stem cells. If an enhanced MSC population is desired, it takes about 2-3 weeks in the laboratory to culture the cells. It is possible that an off-the-shelf stem cell product could be developed based on donor MSC, but it would carry high production costs. By comparison, a TBI therapy that relies on small molecules, such as the NCEs, can be on the shelf for immediate treatment with no dose limitations, at a much lower cost of production, and with easy administration.
Since TBI is a complex multi-system condition, treatments are needed that target simultaneously various biochemical pathways that occur at different time points post the initial brain injury. A combination therapy is most likely to be successful at attenuating and even preventing secondary TBI-associated neuronal death and neurological dysfunction (3).
References
- Nichols JE, Niles JA, Dewitt D, Prough D, Parsley M, Vega S, Cantu A, Lee E, Cortiella J. Neurogenic and neuro-protective potential of a novel subpopulation of peripheral blood-derived CD133+ ABCG2+CXCR4+ mesenchymal stem cells: development of autologous cell-based therapeutics for traumatic brain injury. Stem Cell Res Ther. 2013 4(1):3.
- Zhang ZX, Guan LX, Zhang K, Zhang Q, Dai LJ. A combined procedure to deliver autologous mesenchymal stromal cells to patients with traumatic brain injury. Cytotherapy. 2008;10(2):134-139.
- Harel A., New approach to brain trauma by chemical combination, 2nd international conference: Neurology & Therapeutics, 2013, Chicago, USA poster