Вы здесь
Storage of Mesenchymal Stem/Stromal cells in family stem cell banks: What do they offer?

Mesenchymal stem/stromal cells (MSC) are being widely studied in research and in clinical trials. Their useful properties include the ability to modulate the patient's immune system, promote cell growth, and to differentiate into more specialized cell types. This amazing potential has been explored in a large scope of diagnoses such as graft versus host disease (GvHD), rheumatoid arthritis, cardiomyopathy, liver cirrhosis, Alzheimer's, spinal cord injury, chronic wounds, and diabetes among many others [1,2].
In 2013 more than 5600 articles were published (accessed via PubMed) with MSC as main subject, and during the present year around 1000 papers were already published under the same subject. Demonstrating the medical potential around MSC, 372 clinical trials are ongoing and 17% of them use umbilical cord tissue (UC) as the only MSC source (see Figure).
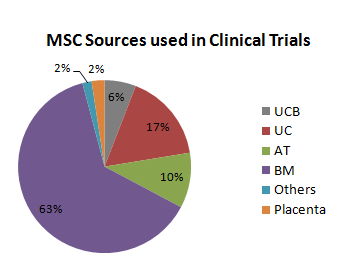 Figure 1: MSC sources listed on ClinicalTrials.gov during March 2014. Keyword search criteria were: umbilical cord blood (UCB), umbilical cord (UC), adipose tissue (AT), bone marrow (BM), placenta, and mesenchymal stem cells. The group named "others" includes MSC from endometrium, synovium and dental pulp.
Figure 1: MSC sources listed on ClinicalTrials.gov during March 2014. Keyword search criteria were: umbilical cord blood (UCB), umbilical cord (UC), adipose tissue (AT), bone marrow (BM), placenta, and mesenchymal stem cells. The group named "others" includes MSC from endometrium, synovium and dental pulp.
Since the number of MSC decreases with a person's age [3], neo-natal sources such as umbilical cord blood (UCB), umbilical cord tissue (UC), or placenta are being studied to replace adult ones such as bone marrow (BM), adipose tissue (AT) or dental pulp (DP).
The isolation of MSC from neo-natal sources have several advantages over adult sources: the procedure is completely non-invasive and has high success rates associated [4,5]. Moreover recent studies indicate that MSC collected from neo-natal sources have higher proliferative abilities than MSC isolated from adult ones [6].
Many stem cell banks around the world now offer the service of collecting MSC from umbilical cord tissue. Despite being technically possible, the storage of MSC from cord blood is not commercially offered because the isolation rates are really low [7]. From a stem cell bank point of view, cord blood is stored exclusively due to its content of blood-forming (hematopoietic) stem cells, and cord tissue is stored due to its MSC content.
When family cord blood banks offer the service of storing MSC from umbilical cord tissue (UC), they are not always selling the same thing. This is an area where most parents are completely unaware of the differences in laboratory procedures and the medical potential of the final product.
Within the family-oriented stem cell bank context, three main services are being offered nowadays: (1) Full or segmented cord storage, (2) Explants processing, and (3) Digestion processing. As the processing and storage methods have a major impact on the quantity and quality of the cellular yield [8-10], it is extremely important for parents to have full knowledge around what type of services are being offered.
The simplest service available is Full/segmented Cord Storage. The umbilical cord is collected at birth, washed several times at the lab, immersed within an appropriate solution carrying a cryoprotectant agent, and then cryopreserved. The cryoprotectant agents are molecules that protect tissues and cells from freezing damages. Sometimes the cord is cut into segments, but it is basically the same procedure. With Full/segmented Cord Storage, MSC are not isolated from the cord. The hope is that they can someday be retrieved from the cord tissue with good cell yield and viability.
There are two basic methods for processing the umbilical cord tissue to isolate the MSC: the Explants method or the Digestion method.
The Explants method starts by mincing the umbilical tissue into tiny pieces and growing them in a culture medium. This allows the MSC to be isolated because they adhere to the surface of the culture dishes. However, it is important to know that most stem cell banks do not fully perform the cell isolation with Explants method. After mincing the cord into pieces, they do not wait for cells to culture and adhere, instead the cord pieces are placed in a cryoprotectant agent and immediately cryopreserved. Under these conditions the explants processing is quite similar to the Full/segmented storage.
The Digestion method relies on the action of enzymes to break up the collagen matrix of the cord tissue. The enzyme digestion is followed by several steps to wash, filter, and centrifuge the separated cells. This method results in a solution that carries fully isolated MSC. The solution is then cryopreserved. At the end of Digestion processing the cells are completely ready for further operations, if needed.
The Table summarizes the available UC-MSC storage services for parents. From a stem cell bank perspective, Full/segmented cord storage as well as Explants processing service are both quite simple and fast [11], and have a low level of "substantial manipulation" (to quote FDA terminology) [12]. The digestion processing is more prolonged, requires many steps, and has a higher degree of manipulation because of the use of enzymatic solutions. The reagents in the digestion method are also more expensive. However, a parent perspective should be about quality control, and when the MSC are not isolated, it is difficult to know their number, identity, and viability or proliferation properties. Only in Digestion processing do we know how many cells of what quality were harvested.
Table: Comparison of UC-MSC storage offered in stem cell banks
| Characteristics | Full/segmented Cord | Explants Processing | Digestion Processing |
| State of MSC | MSC trapped in UC | MSC trapped in UC | MSC fully isolated |
| Time consuming | Lower | Lower | Higher |
| Technical difficulty | Lower | Lower | Higher |
| Degree manipulation | Lower | Lower | Higher |
| Process Cost | Lower | Lower | Higher |
| Quality control | Harder | Harder | Easier |
| Integration of process | Harder | Harder | Easier |
Summarizing, parents should be aware that, before they can be used in clinical trials, the cryopreserved MSC will have to be further processed after thaw for patient application. Only in Digestion processing do we truly know the cell count, cell properties, and cell viability of the MSC. In Full/segmented cord storage and Explants processing, the MSC are still entrapped within the cord pieces, requiring additional effort post-thaw to isolate the MSC. But even if parents choose the Digestion processing, their cells are still not treatment-ready. Since the number of cells required for MSC therapy is high (usually ranging between 1-6 millions of MSC per Kg of patient's body weight) the MSC collected during the isolation processes are not enough [13] and need always to be expanded in the laboratory before treatment.
Parents who wish to store MSC from cord tissue should look at exactly what kind of services are being offered by the stem cell banks, how they perform the quality control tests, and ultimately what kind of guarantees they offer in case of the sample is released for transplantation purposes.
References
- R.C. Schugar, S.M. Chirieleison, K.E. Wescoe, B.T. Schmidt, Y. Askew, J.J. Nance, J.M. Evron, B. Peault, and B.M. Deasy, High harvest yield, high expansion, and phenotype stability of CD146 mesenchymal stromal cells from whole primitive human umbilical cord tissue J. Biomed. Biotechnol. 2009:789526, Jan. 2009
- R.R. Taghizadeh, K.J. Cetrulo, and C.L. Cetrulo, Wharton's Jelly stem cells: future clinical applications Placenta, 32(4):S311-5, Oct. 2011.
- A.I. Caplan, Why are MSCs therapeutic? New data: new insight J. Pathol. 217(2):318-24, Jan. 2009.
- C. De Bruyn, M. Najar, G. Raicevic, N. Meuleman, K. Pieters, B. Stamatopoulos, A. Delforge, D. Bron, and L. Lagneaux, A rapid, simple, and reproducible method for the isolation of mesenchymal stromal cells from Wharton's jelly without enzymatic treatment Stem Cells Dev., 20(3):547-57, Mar. 2011.
- C.-Y. Fong, A. Subramanian, A. Biswas, K. Gauthaman, P. Srikanth, M. P. Hande, and A. Bongso, Derivation efficiency, cell proliferation, freeze-thaw survival, stem-cell properties and differentiation of human Wharton's jelly stem cells Reprod. Biomed. Online, 21(3):391-401, Sep. 2010.
- R. Hass, C. Kasper, S. Böhm, and R. Jacobs, Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC Cell Commun. Signal. 9(1):12, Jan. 2011.
- M. Secco, E. Zucconi, N.M. Vieira, L.L.Q. Fogaça, A. Cerqueira, M.D.F. Carvalho, T. Jazedje, O.K. Okamoto, A.R. Muotri, & M. Zatz, Multipotent stem cells from umbilical cord: cord is richer than blood! Stem Cells, 26(1):146-50, Jan. 2008.
- A. Iftimia-Mander, P. Hourd, R. Dainty, and R.J. Thomas, Mesenchymal Stem Cell Isolation from Human Umbilical Cord Tissue: Understanding and Minimizing Variability in Cell Yield for Process Optimization Biopreserv. Biobank., 11(5):291-298, Oct. 2013.
- P. Salehinejad, N.B. Alitheen, A.M. Ali, A.R. Omar, M. Mohit, E. Janzamin, F.S. Samani, Z. Torshizi, and S.N. Nematollahi-Mahani, Comparison of different methods for the isolation of mesenchymal stem cells from human umbilical cord Wharton's jelly In Vitro Cell. Dev. Biol. Anim. 48(2):75-83, Feb. 2012.
- Y.-F. Han, R. Tao, T.-J. Sun, J.-K. Chai, G. Xu, and J. Liu, Optimization of human umbilical cord mesenchymal stem cell isolation and culture methods Cytotechnology 51, Jan. 2013.
- A. Petsa, S. Gargani, A. Felesakis, N. Grigoriadis, and I. Grigoriadis, Effectiveness of protocol for the isolation of Wharton's Jelly stem cells in large-scale applications In Vitro Cell. Dev. Biol. Anim. 45(10):573-6, Dec. 2009.
- The Parliament Council of the Union, Regulation (EC) No.1394/2007 of the European Parliament and of the Coucil of 13 November 2007 no. 1394, 2007.
- N. Tsagias, I. Koliakos, V. Karagiannis, M. Eleftheriadou, and G.G. Koliakos, Isolation of mesenchymal stem cells using the total length of umbilical cord for transplantation purposes Transfus. Med. 21(4):253-61, Aug. 2011.


