Jesteś tutaj
Transforming Transplants with Deceased Donor Stem Cells
 NJ Sharing Network recently announced the launch of a new Personalized Transplant Medicine Institute (PTMI). In what represents a significant advancement in transplant medicine, PTMI will be dedicated to the research and development of innovations aimed at increasing the number of successful transplants of organs, tissues, and stem cells. The PTMI’s multifaceted approach will include personalized genomics, assay development, living kidney donation, and regenerative medicine, all of which will transform ‘transplantology’ as it is known today. This initiative will be led by Dr. Prakash Rao, VP of Diagnostics & Research Operations and Director of the Transplant Laboratory at NJ Sharing Network.
NJ Sharing Network recently announced the launch of a new Personalized Transplant Medicine Institute (PTMI). In what represents a significant advancement in transplant medicine, PTMI will be dedicated to the research and development of innovations aimed at increasing the number of successful transplants of organs, tissues, and stem cells. The PTMI’s multifaceted approach will include personalized genomics, assay development, living kidney donation, and regenerative medicine, all of which will transform ‘transplantology’ as it is known today. This initiative will be led by Dr. Prakash Rao, VP of Diagnostics & Research Operations and Director of the Transplant Laboratory at NJ Sharing Network.
In 2014, NJ Sharing Network took a huge leap forward by becoming one of the first Organ Procurement Organizations to start a regenerative medicine program. Since that time, the organization has successfully procured, isolated, and grown “adult” stem cells from research-consented deceased donors.
Of particular interest are the cells procured from deceased donor bone marrow. Our recent experiments confirm that cell count, viability, percentage of hematopoietic progenitor cells, and the ability to form cell colonies from deceased donor bone marrow is comparable to living donor bone marrow.
Above we display photos of cell colonies grown from the bone marrow of research-consented deceased donors. The Colony Forming Unit (CFU) assay was carried out for Hematopoietic Stem Cells (HSC) from the iliac crest and femur. First, we determined the volume of bone marrow buffy coat required to plate cells at a density of 1.25 x 104 cells per well. Contamination by red blood cells was minimized by sedimentation over HetaSepTM (Stemcell Technologies Inc., Vancouver, BC, Canada). A 1 ml working solution containing 10X cell suspension was prepared using Iscove's Modified Dulbecco's Media (IMDM) (Stemcell Technologies Inc., Vancouver, BC, Canada). To set up the CFU assay, 400 µl of the 10X working cell suspension was added to 4 ml of pre-aliquoted and thawed MethoCultTM media (Stemcell Technologies Inc., Vancouver, BC, Canada). The MethoCultTM/cell mixture was dispensed in 1 ml aliquots into the pre-labeled SmartDishTM in triplicate. Sterile water was added to the space between the wells to help maintain humidity during incubation. The SmartDishTM containing the MethoCultTM/cell mixture and water was incubated in a CO2 incubator set at 37°C and 5% CO2 for 14-16 days. After the incubation period, each distinct colony was counted and identified by its specific morphological characteristics using an inverted phase contract microscope. The photos are from a digital camera attached to the microscope.
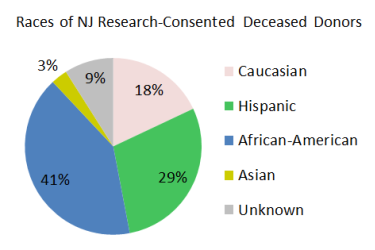
Currently, it is more difficult for minority and mixed-race patients to get life-saving stem cell transplants because there is a large disparity in their ability to find matching bone marrow donors. By comparison, the majority of organ donors in New Jersey belong to the racial groups that are sought after for bone marrow donation. We plan to target bone marrow procurements from minority and mixed-race deceased donors to help close the gap in transplant access for these racial groups.
Advantages of Deceased Donor Bone Marrow:
- Deceased donors are routinely phenotyped for human leukocyte antigens and evaluated for the presence of any potential infectious diseases; they represent a safe, accessible, and an economically viable source of stem cells.
- Bone marrow procurement from deceased donors can be routinely performed at the same time as organ and tissue procurement.
- With deceased donors, bone marrow may be procured directly from the bones rather than the iliac crest or sternum.
- The pain and/or discomfort experienced by living bone marrow donors is non-existent.
- Deceased donor bone marrow could be cryopreserved in public banks similar to the way umbilical cord blood is stored in an inventory for patients seeking a matching donor.
NJ Sharing Network is in the process of publishing the results of our promising research on stem cells from deceased donor sources, including bone marrow. Through the PTMI, we hope to make these additional stem cells sources available for further research and eventually patient treatment.





 We can’t help but ask ourselves, “How cool would it be if one day, we could tell a donor family that their deceased loved one was not only able to save and enhance lives through organ and tissue donation, but also able to treat cancer?”
We can’t help but ask ourselves, “How cool would it be if one day, we could tell a donor family that their deceased loved one was not only able to save and enhance lives through organ and tissue donation, but also able to treat cancer?”