Usted está aquí
Cerebral Palsy Cell Therapy Comparison
2022 Update: Download a spreadsheet holding all clinical trials of cell therapy for Cerebral Palsy or Autism registered 2017 - 2021.
In this review we graph the results of four different stem cell therapies for cerebral palsy against each other. Specifically we compare the degree of improvement that each therapy has on Gross Motor Function Measure (GMFM) score.
The purpose of this review is two-fold. First, we wish to illustrate that multiple published studies have proven that stem cell therapy significantly improves GMFM scores for cerebral palsy patients. Second, we want to start a conversation about the differences between the studies, in hopes that it will inspire researchers to adjust their treatment protocols to converge on methods that are more directly comparable and more effective for patients. We do not intend for anyone to interpret this graph as showing one study is “better” than the others, because there are many differences between these studies and no two are directly comparable.
In this Table we list the four data sets that are graphed with links to their three publications. All of these publications are the outcome of clinical trials that conducted controlled studies. The cord blood studies were also double-blind. All of these publications demonstrated that stem cell therapy produced a statistically significant improvement in GMFM scores for cerebral palsy patients.
Group Leader | Publication | Cell Therapy Type |
Minyoung Kim, MD PhD1 | allogeneic CB-MNC | |
Joanne Kurtzburg, MD3 | autologous CB-MNC | |
Yihua An, MD4 | autologous BM-MNC | |
Yihua An, MD4 | autologous BM-MSC |
Terminology in Table:
Allogeneic: Cells from a donor, obtained from inventory in a public bank
Autologous: Cells from the patient
BM: bone marrow
CB: cord blood
MNC: Mono-Nuclear Cells
MSC: Mesenchymal Stromal Cells
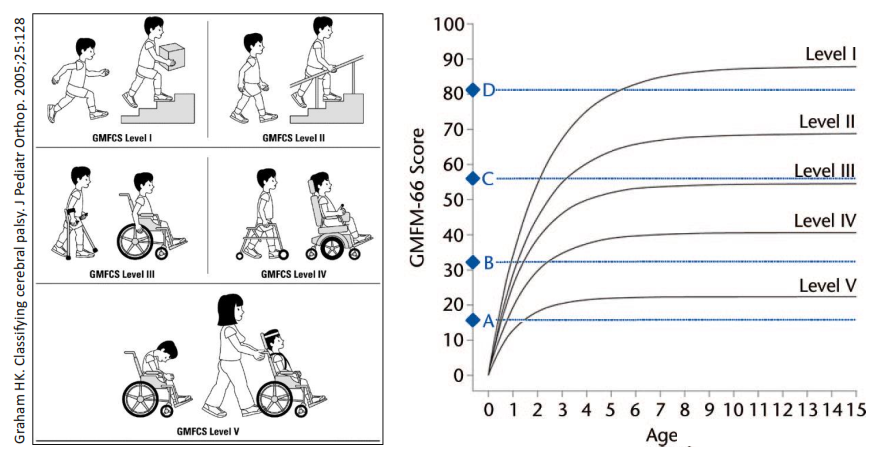
The figure below illustrates the fundamental problem of trying to quantify the impact of any therapy for cerebral palsy patients. First, the patients vary in degree of disability. Second, over time their gross motor function will improve without intervention, up to a plateau that occurs between ages 3 and 6, depending on their functional level. Against this backdrop, the only way that measured GMFM improvements can be considered significant is if they are bigger than predicted for the patient group or bigger than the improvements in a properly matched control group.
The bar graph below displays the difference between the GMFM improvement of the cell therapy group minus the improvement of the control group for each study in our table. We displayed the best result from each research group, where possible. For allo-CB-MNC, we display the result for children under age 3, and for auto-CB-MNC we display the result for high-dose patients. There is one previous meta-analysis5 by Novak et al. 2016 that also compared the GMFM improvements in five studies of stem cell therapy for cerebral palsy.
There are numerous differences between these research groups, both profound and subtle. The variables between the study groups include: type of cells used for cell therapy, cell therapy route of administration, cell dose, type of simultaneous rehabilitation, variability of rehabilitation within and among study arms, the ages and functional levels of patients in the study, and the duration of follow up.
The only scores in this graph that can be directly compared to each other are the two arms of Dr. An’s study, which found that cell therapy with auto-BM-MSC yielded larger GMFM improvements than auto-BM-MNC, and this difference was statistically significant.
An important practical question is how many stem cells are needed to deliver effective cell therapy for cerebral palsy? The main finding of Dr. Kurtzberg’s cord blood study was that “high dose” patients who received over 20 million cells per kg via IV had significantly more improvement. Dr. Kim’s group also found significantly more improvement with higher cord blood doses via IV and better HLA matches, and their dose range extended above 100 million cells per kg. However, cord blood doses that high are difficult to achieve unless the patients are small and there is access to large donated cord blood units for those patients who did not have a good collection of their own cord blood. By comparison, the intrathecal delivery method used by Dr. An’s group achieves significant therapeutic results with only 4 million cells per kg total, so that any patient with access to a small amount of their own bone marrow or cord blood would have access to therapy. However, intrathecal cell delivery has the highest observed rate and known risk of serious adverse events, so it is necessary to prove that this method can be delivered safely in multiple clinical settings.
While all of these studies have proven that stem cell therapy is beneficial for cerebral palsy patients, it will take more controlled studies to test these therapeutic approaches against each other, and to clarify the role of rehabilitation during cell therapy. We conclude with a few provocative thoughts:
- If intrathecal delivery can be established as a safe standard, then cerebral palsy patients could receive multiple rounds of cell therapy free of concerns about obtaining high cell doses.
- If the intensive in-patient rehabilitation that is provided by Dr. Kim’s group contributes to the patient response, can that program of physical and occupational therapy be exported to the west?
- If MSC therapy performs better than MNC, those MSC can be sourced from umbilical cord tissue so the patients do not have to undergo lumbar puncture.
References
- Min K, Song J, Kang JY, Ko J, Ryu JS, Kang MS, Jang SJ, Kim SH, Oh D, Kim MK, Kim SS & Kim M. Umbilical Cord Blood Therapy Potentiated with Erythropoietin for Children with Cerebral Palsy: A Double-blind, Randomized, Placebo Controlled Trial. 2013; Stem Cells, 31(3):581-591.
- Kang M, Min K, Jang J, Kim SC, Kang MS, Jang SJ, Lee JY, Kim SH, Kim MK, An SSA, & Kim, M. Involvement of Immune Responses in the Efficacy of Cord Blood Cell Therapy for Cerebral Palsy. 2015; Stem cells and development, 24(19):2259-2268.
- Sun JM, Song AW, Case LE, Mikati MA, Gustafson KE, Simmons R, Goldstein R, Petry J, McLaughlin C, Waters‐Pick B, Chen LW, Wease S, Blackwell B, Worley G, Troy J, & Kurtzberg J. Effect of Autologous Cord Blood Infusion on Motor Function and Brain Connectivity in Young Children with Cerebral Palsy: A Randomized, Placebo‐Controlled Trial. 2017; Stem Cells Translational Medicine, 6(12):2071-2078
- Liu X, Fu X, Dai G, Wang X, Zhang Z, Cheng H, Zheng P, & An Y. Comparative analysis of curative effect of bone marrow mesenchymal stem cell and bone marrow mononuclear cell transplantation for spastic cerebral palsy. 2017; Journal of Translational Medicine, 15:48
- Novak I, Walker K, Hunt RW, Wallace E, Fahey M, Badawi N. Stem cell interventions for people with cerebral palsy: Systematic review with meta-analysis. 2016; Stem Cells Translational Medicine, 5(8):1014–1025.
- Rosenbaum PL, Walter SD, Hanna SE, et al. Prognosis for gross motor function in cerebral palsy: creation of motor development curves. JAMA. 2002;288:1357–1363
- Hanna SE, Bartlett DJ, Rivard LM, & Russell DJ. Reference Curves for the Gross Motor Function Measure: Percentiles for Clinical Description and Tracking Over Time Among Children With Cerebral Palsy. 2008; Physical Therapy 88(5):596–607
- CanChild Resources: Gross Motor Function Measure (GMFM) accessed on-line
- Ko J & Kim M. Inter-rater Reliability of the K-GMFM-88 and the GMPM for Children with Cerebral Palsy. 2012; Ann Rehabil Med. 36(2):233–239.



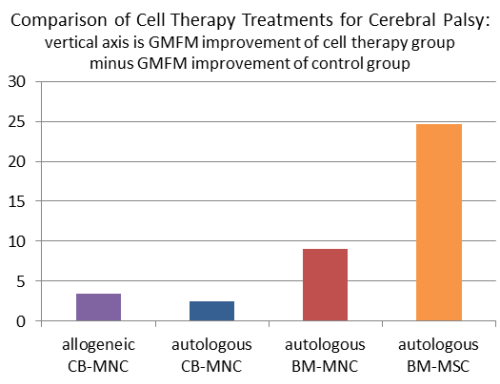 An important caveat about the scores in this graph is that the two scores on the right from Dr. An’s group were measured on the GMFM-88 scale, whereas the two scores on the left from the cord blood studies by Dr. Kim’s group and Dr. Kurtzberg’s group used the GMFM-66 scale. The GMFM-66 questionnaire is a subset of the GMFM-88 questionnaire that is interpreted with the help of a computer program6-8. Research has shown that GMFM-88 scores are highly correlated to GMPM measures, but in absolute values the scoring scales differ9.
An important caveat about the scores in this graph is that the two scores on the right from Dr. An’s group were measured on the GMFM-88 scale, whereas the two scores on the left from the cord blood studies by Dr. Kim’s group and Dr. Kurtzberg’s group used the GMFM-66 scale. The GMFM-66 questionnaire is a subset of the GMFM-88 questionnaire that is interpreted with the help of a computer program6-8. Research has shown that GMFM-88 scores are highly correlated to GMPM measures, but in absolute values the scoring scales differ9.