Usted está aquí
Cleveland Cord Blood Center Research on Type 1 Diabetes
 Approximately 1.25 million American children and adults have Type 1 Diabetes, with about 70,000 children diagnosed with the disease each year. That number is rising by 3 to 5 percent annually in developing countries, suggesting that changing environmental factors may contribute to the disease.
Approximately 1.25 million American children and adults have Type 1 Diabetes, with about 70,000 children diagnosed with the disease each year. That number is rising by 3 to 5 percent annually in developing countries, suggesting that changing environmental factors may contribute to the disease.
Mechanism of Type 1 Diabetes
Type 1 Diabetes is a consequence of the individual’s immune system destroying the insulin-producing beta cells (islet) in the pancreas. By the time patients are diagnosed with Type 1 Diabetes, a majority of their beta cells have already been destroyed. Hence current treatments focus on disease management. A major challenge is to identify treatments that reset the immune response, and result in long-term beta cell preservation.
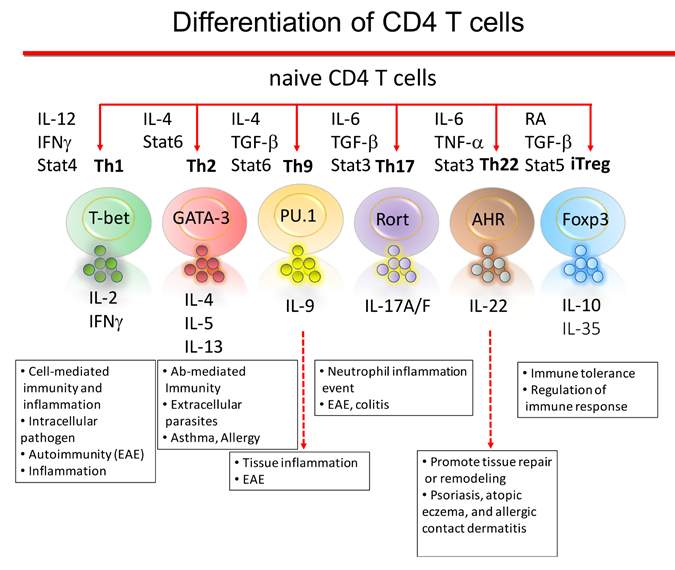
Patients that are developing Type 1 Diabetes do not have a normal number and function of regulatory T cells in their blood. Regulatory T cells (Tregs) are a type of white blood cell that is a critical immune regulator to maintaining tolerance and limiting auto-immune reactions.1-3
Animal studies show Tregs that migrate into an inflammatory region inhibit auto-immune responses.4,5 One limitation to the generation of Tregs for effective treatment of Type 1 Diabetes is that veryhttps://parentsguidecordblood.org/en/news/cleveland-cord-blood-center-re... few Tregs that can be obtained from adolescent patients that are developing the disease. A second limitation is the inability to grow Tregs outside the body to generate a larger number of cells while keeping their immune control function intact.
Pre-clinical Studies
Researchers at the Cleveland Cord Blood Center (CCBC) are studying T cells from the umbilical cord blood of healthy newborn babies. The T cells from newborn babies are unique in that they have very strong T regulatory capacity in order to provide immune tolerance between the baby and the mother during pregnancy. The CCBC researchers are taking the cell biology knowledge gained from investigating neonatal immune tolerance and applying it to treat patients with Type 1 Diabetes.
Currently CCBC researchers are conducting pre-clinical studies to verify that Tregs from umbilical cord blood can safely suppress the abnormal T cell attack on the pancreas islet cells that occurs in Type 1 Diabetes patients. It is anticipated that this intervention would be most effective when the patient is recently diagnosed. If the Tregs can stop the auto-immune attack on the patient’s insulin-producing islet cells in the pancreas, this will allow beta cell recovery, and improvement or resolution of the diabetes. See below for planned clinical trials.
Clinical Studies
Eventually, CCBC researchers envision a treatment approach to Type 1 Diabetes that would proceed as follows:
- Identify an umbilical cord blood unit that is HLA matched to the Type 1 Diabetes patient;
- Isolate stem cells from the cord blood unit;
- Generate induced Tregs (iTregs) from the cord blood stem cells;
- Grow the iTregs for three weeks in sterile culture conditions uniquely developed by the research team to maintain T regulatory function;
- Infuse the Type 1 Diabetes patient with these expanded umbilical cord blood Tregs.
References
- Lindley S, Dayan CM, Bishop A, Roep BO, Peakman M, Tree TI. Defective suppressor function in CD4(+)CD25(+) T-cells from patients with type 1 diabetes. Diabetes 2005 Jan; 54(1): 92-99.
- Brusko TM, Wasserfall CH, Clare-Salzler MJ, Schatz DA, Atkinson MA. Functional defects and the influence of age on the frequency of CD4+ CD25+ T-cells in type 1 diabetes. Diabetes 2005 May; 54(5): 1407-1414.
- Cretney E, Kallies A, Nutt SL. Differentiation and function of Foxp3(+) effector regulatory T cells. Trends Immunol 2013 Feb; 34(2): 74-80.
- Cantor J, Haskins K. Effector function of diabetogenic CD4 Th1 T cell clones: a central role for TNF-alpha. J Immunol 2005 Dec 1; 175(11): 7738-7745.
- Tonkin DR, Haskins K. Regulatory T cells enter the pancreas during suppression of type 1 diabetes and inhibit effector T cells and macrophages in a TGF-beta-dependent manner. Eur J Immunol 2009 May; 39(5): 1313-1322.



 Jeong-su Do, PhD, currently holds a post-doctoral research fellowship at the Cleveland Clinic, working in collaboration with the Cleveland Cord Blood Center’s research and development laboratory. Dr. Do received his BS degree, MSc degree, and PhD from Jeonju University in South Korea. Dr. Do and his co-investigators have published over 31 papers, including more than 14 peer-reviewed papers. Launched in 2008, the Cleveland Cord Blood Center (CCBC) collects, processes, stores, and distributes umbilical cord blood for stem cell transplants as well as research in advanced cellular therapy. Located in Warrensville Heights OH, CCBC is a public cord blood bank and a not-for-profit 501(c)(3) organization. For more information, visit
Jeong-su Do, PhD, currently holds a post-doctoral research fellowship at the Cleveland Clinic, working in collaboration with the Cleveland Cord Blood Center’s research and development laboratory. Dr. Do received his BS degree, MSc degree, and PhD from Jeonju University in South Korea. Dr. Do and his co-investigators have published over 31 papers, including more than 14 peer-reviewed papers. Launched in 2008, the Cleveland Cord Blood Center (CCBC) collects, processes, stores, and distributes umbilical cord blood for stem cell transplants as well as research in advanced cellular therapy. Located in Warrensville Heights OH, CCBC is a public cord blood bank and a not-for-profit 501(c)(3) organization. For more information, visit