Usted está aquí
Growing use of Newborn Stem Cells in Clinical Trials
Many parents are aware that there are stem cells in cord blood, but in fact most of the blood and tissues in the afterbirth are rich in what collectively are known as perinatal stem cells. These are a varied population of cells.
- Cord blood is rich in blood-forming (hematopoietic) stem cells and is prized as a source to treat disorders and cancers of the blood, both through conventional stem cell transplants as well as the newer “immunotherapy” treatments for cancer.
- The Wharton’s Jelly that fills the tissue of the umbilical cord is rich in mesenchymal stem cells (MSC) that can differentiate to become bone, cartilage and muscle (among others). These are valuable for their potential in orthopedic and cardiac therapies, as well as other therapies where the MSC act to suppress inflammation and auto-immune reactions. The MSC can also be derived from cord blood, but with much lower efficiency than using other perinatal tissues as a source of MSC.
- The sheath that lines the placenta and umbilical cord, known as the amniotic membrane, holds a mixture of MSC and cells that are precursors to skin (epithelial stem cells).
- The placenta and chorion are also a rich source of a mixed population of stem cells, especially MSC.
Since the first cord blood transplant in 1988, public cord blood banks have built an inventory of cord blood donations that can be used to save the lives of patients seeking a matching donor. Over the following 28 years there have been over 35,000 cord blood transplants world-wide (Ballen Verter Kurtzberg 2015). In these cell therapies the cord blood stem cells are un-manipulated and are intended to replace cells that the patient lost during chemotherapy.
Over the past decade a new branch of medicine has evolved in which perinatal stem cells are used in advanced cell therapies, where either the cells are manipulated before being incorporated into therapies, and/or they are expected to perform a function beyond the replacement of lost cells. Regenerative medicine is defined (Mason & Dunnil 2008) as therapy that "replaces or regenerates human cells, tissue or organs, to restore or establish normal function". The scope of advanced cell therapies is even broader than the field of regenerative medicine. We describe a few examples.
- When unmanipulated perinatal cells are given to patients to promote healing from neurologic disorders of the brain such as encephalopathy, cerebral palsy, autism, stroke, etc., those are advanced cell therapies.
- When MSC from perinatal tissues are administered to suppress auto-immune disorders such as Graft-versus-Host disease (GvHD) or multiple sclerosis, that qualifies as advanced cell therapies.
- When special types of immune-reactive cells are extracted from cord blood and/or the stem cells are grown in culture before a cord blood transplant, those are also advanced cell therapies.
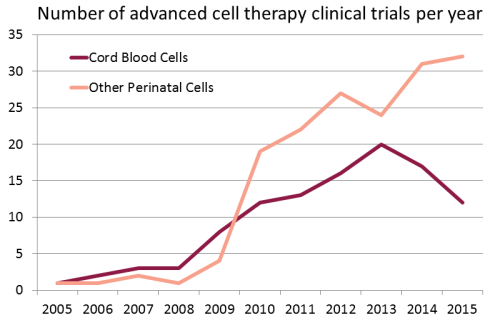 We have compiled a database of all clinical trials worldwide over the years 2005 - 2015 that used perinatal cells to perform advanced cell therapies. There are a total of 278 trials in this category. We applied a definition of advanced cell therapy consistent with the European Medicines Agency (EMA) terminology for Advanced Therapy Medicinal Product (ATMP). We searched a dozen international registries of clinical trials, starting with the USA registry ClinicalTrials.gov, but also including the WHO international registry, the EU clinical trials register, and the national registries of Japan, China, South Korea, Australia and New Zealand, India, Iran, Germany, and the Netherlands.
We have compiled a database of all clinical trials worldwide over the years 2005 - 2015 that used perinatal cells to perform advanced cell therapies. There are a total of 278 trials in this category. We applied a definition of advanced cell therapy consistent with the European Medicines Agency (EMA) terminology for Advanced Therapy Medicinal Product (ATMP). We searched a dozen international registries of clinical trials, starting with the USA registry ClinicalTrials.gov, but also including the WHO international registry, the EU clinical trials register, and the national registries of Japan, China, South Korea, Australia and New Zealand, India, Iran, Germany, and the Netherlands.
Some pharmaceutical companies have invested in clinical trials with perinatal stem and progenitor cells. Our second figure displays the number of advanced cell therapy clinical trials per year sponsored by industry that employ perinatal cells. Over the past decade, 105 clinical trials of advanced cell therapy with perinatal cells have been sponsored by 30 companies in 11 countries.
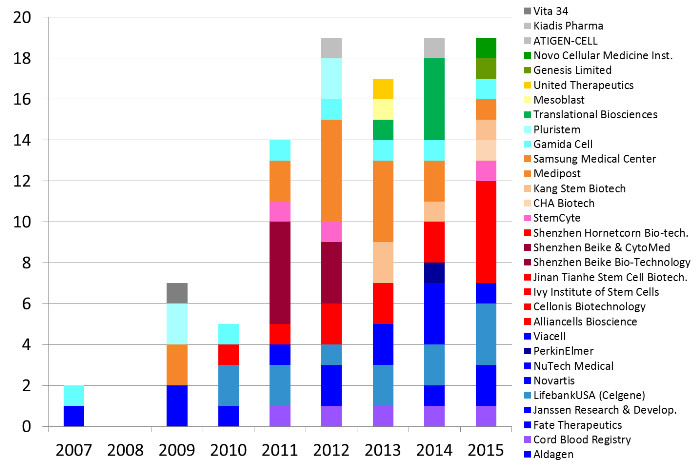
Some companies are notable for sponsoring numerous clinical trials of a particular treatment modality. Below we describe those companies that have sponsored 5 or more clinical trials.
Companies based in the United States (colored shades of dark blue through purple) have sponsored 37 trials of advanced cell therapies with perinatal cells. LifebankUSA (until recently part of Celgene) has sponsored 14 clinical trials, mostly devoted to trials with placental cells but also exploring immunotherapy with cord blood cells. Next in the United States are three companies that have 5 trials each: Cord Blood Registry funds trials of regenerative medicine with cord blood, Fate Therapeutics until recently funded trials that incubated cord blood stem cells prior to transplant, and NuTech Medical has explored applications of amniotic fluid and membrane.
Companies based in China (colored shades of red) have sponsored 21 trials of advanced cell therapies with perinatal cells. Shenzhen Beike and collaborators have sponsored 8 trials, primarily using MSC derived from cord tissue, sometimes in combination with cord blood stem cells. This does not include trials in Taiwan (colored pink).
In South Korea the industry sponsored trials of perinatal cells are dominated by Medipost and collaborators (colored shades of orange), who together account for 16 trials of advanced cell therapies, every one of them using MSC derived from cord blood.
The last company with 5 or more trials is Gamida Cell in Israel (colored shades of light blue) that has sponsored 7 clinical trials in which cord blood stem cells are manipulated to improve cord blood transplants. The remaining geographic regions holding companies that sponsored perinatal trials are Australia (shades of yellow and tan), Latin America (shades of dark green) and the EU (shades of gray).
The advanced perinatal cell therapies sponsored by industry could best be described as existing in silos. Each company has a treatment modality that they are pushing through a pipeline of trials, with the eventual goal of obtaining regulatory approval in their home country. One company may focus on MSC derived from cord tissue while another focuses on MSC derived from cord blood. There is unlikely to ever be a head-to-head comparison of these MSC therapies from different cell sources until they reach the marketplace. One can bemoan the lack of head-to-head comparisons that would be valuable for patients, but this is the reality of how companies must function in order to finance the process of translating a therapy from bench to bedside.
We predict continued growth in the field of advanced cell therapies with newborn stem cells, as more clinicians embrace the ease of obtaining relatively large quantities of immunologically naive stem and progenitor cells from perinatal sources.


