Sie sind hier
The Cells4Life Advantage
Any bank can store your baby's cord blood, but only Cells4Life can save 3X more stem cells.
 The single most important factor determining how successful your baby’s cord blood could be in therapy is the number of viable stem cells it contains at the point of treatment. A sample that’s useful for a child may be inadequate for that same person as an adult. Or it may only contain enough cells for one therapy, rather than several.
The single most important factor determining how successful your baby’s cord blood could be in therapy is the number of viable stem cells it contains at the point of treatment. A sample that’s useful for a child may be inadequate for that same person as an adult. Or it may only contain enough cells for one therapy, rather than several.
Most cord blood banks don’t like to talk about it, but many of the industry standard processing methods that they use lose significant numbers of stem cells. These losses occur at each step - after processing, freezing, and finally thawing the sample when you come to use it.
That’s why scientists at Cells4Life developed CellsPlus™ to process their children’s cord blood.
In a peer-reviewed scientific study, our patented TotiCyte™ technology – the engine behind CellsPlus™ – was tested head-to-head against other major cord blood processing systems used in the US, including AXP, HES, MacoPress Smart, and Sepax 2.
The results are clear: CellsPlus™ delivers up to 3 TIMES more viable stem cells at the point of therapy1. This is the number that truly matters - the healthy, functional cells that are ready for treatment after being thawed.
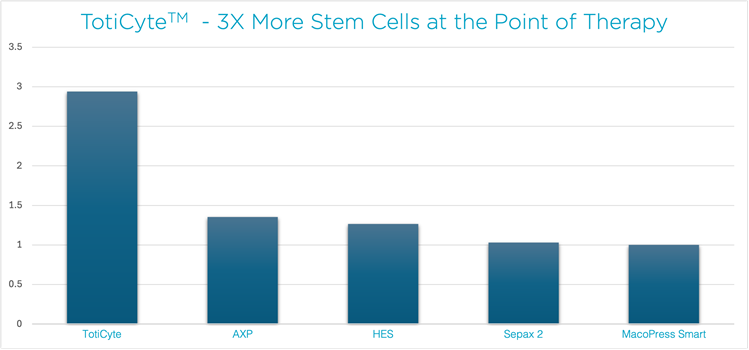
Figure 1: TotiCyteTM post-thaw viable & functional CD34+ stem cell recovery compared to other processing methods
This superior yield means:
- That the sample you store today has a far greater chance of being truly effective, not just for your baby when they are a child, but as full-grown adult.
- More cells may mean not just one, but possibly multiple stem cell treatments from the same sample.
- If you’re choosing delayed cord clamping, our system is efficient enough that even a slightly smaller initial collection can yield the same, or even more, usable cells than a larger sample processed elsewhere.
A Revolutionary First – Stem Cells for Mom
 The birth of your baby provides an opportunity to help secure their future health, and now, Mom’s as well.
The birth of your baby provides an opportunity to help secure their future health, and now, Mom’s as well.
Unique to Cells4Life, Maternal Stem Cell Banking allows you, for the first time, to preserve your own powerful mesenchymal stem cells from the maternal side of the placenta – the decidua.
These maternal cells are a perfect 100% genetic match for you with zero risk of rejection. Research is exploring their potential to treat autoimmune conditions like MS, support cardiovascular health, and aid aid in regulating the immune system2-4.
Beyond Cord Blood: The Most Comprehensive Protection Available
While many banks stop at cord blood and cord tissue, regenerative medicine research hasn't. Clinical trials are now investigating the regenerative potential of a wider range of perinatal sources. That’s why Cells4Life gives families the ability to preserve more of these perinatal tissues and stores each one separately to maximize future therapeutic options.
This includes:
- Cord Tissue: A primary source of Mesenchymal Stem Cells (MSCs), which are the focus of thousands of clinical trials for regenerating tissue like cartilage, muscle, and nerves, and treating conditions such as diabetes, MS, and heart disease5-10.
- Placental Cells: An abundant, secondary source of potent MSCs from the chorionic villi, being studied for their unique potential in treating conditions like Crohn's disease, stroke, and
- osteoarthritis11-13.
- Amnion: The amniotic membrane, a healing tissue used for over a century to treat burns and wounds, is now being explored in clinical trials for eye diseases, orthopedics, and more14,15.
Our advanced services are built on a foundation of the highest quality standards and thoughtful practices. All samples are stored in multiple therapeutic units, preserving the potential for several treatments over your baby's lifetime.
Cells4Life's laboratory is AABB accredited and FDA registered - the gold standards for quality and safety in the industry.
You only get one chance to store your baby's stem cells, and if you can give them 3x more stem cells, why wouldn't you?
Visit cells4life.us to get your free Info Kit with all you need to know about cord blood banking with Cells4Life. Alternatively, contact us on (844) 800-0146 or email us at info@cells4life.com.
Who is Cells4Life? Founded in 2002, Cells4Life is a leading international cord blood bank, trusted with hundreds of thousands of samples from families all over the world. This year, we opened a new office to serve families across the United States. But that's not the most important thing you should know about us. The most important thing is why we started. Our company was founded by scientists for a very personal reason: to store their children's umbilical cord stem cells. That personal mission means that every decision we've taken - from the technology we've invented, our systems and processes, to the services we offer - has been guided by a single question: "Is this what we would do for our own family?" |
References
- Drew J, Slaughter R, Klimentov A, Channon WM, Rees C, et al. (2021) TotiCyte, a Paradigm Shift in Stem Cell Isolation and Storage from Umbilical Cord Blood. J Stem Cell Res Dev Ther 7:073.
- Bravo, B, Gallego, MI, Flores, AI, et al. Restrained Th17 response and myeloid cell infiltration into the central nervous system by human decidua-derived mesenchymal stem cells during experimental autoimmune encephalomyelitis. Stem Cell Res Ther 2016; 7:43. https://doi.org/10.1186/s13287-016-0304-5
- Chen K, Bai L, Lu J, et al. Human Decidual Mesenchymal Stem Cells Obtained From Early Pregnancy Improve Cardiac Revascularization Postinfarction by Activating Ornithine Metabolism. Frontiers in Cardiovascular Medicine. 2022; 9:837780. https://doi.org/10.3389/fcvm.2022.837780
- Sadeghi B, Witkamp M, Schefberger D, Arbman A, Ringdén O. Immunomodulation by placenta-derived decidua stromal cells. Role of histocompatibility, accessory cells and freeze–thawing. Cytotherapy. 2023; 25(1):68-75. https://doi.org/10.1016/j.jcyt.2022.10.004
- Russo E, Caprnda M, et al. Umbilical Cord Mesenchymal Stromal Cells for Cartilage Regeneration Applications. Stem Cells Int. 2022; 2022:2454168. https://doi.org/10.1155/2022/2454168
- Bojanic C, To K, Zhang B, Mak C, Khan WS. Human umbilical cord derived mesenchymal stem cells in peripheral nerve regeneration. World J Stem Cells. 2020; 12(4):288-302. https://doi.org/10.4252/wjsc.v12.i4.288
- Pereira T, Armada-da Silva PA, Amorim I, et al. Effects of Human Mesenchymal Stem Cells Isolated from Wharton's Jelly of the Umbilical Cord and Conditioned Media on Skeletal Muscle Regeneration Using a Myectomy Model. Stem Cells Int. 2014; 2014:376918. https://doi.org/10.1155/2014/376918
- Li L, Li J, Guan H, Oishi H, Takahashi S, Zhang C. Human umbilical cord mesenchymal stem cells in diabetes mellitus and its complications: applications and research advances. Int J Med Sci. 2023; 20(11):1492-1507. https://doi.org/10.7150/ijms.87472
- Jamali F, Aldughmi M, Atiani S, Al-Radaideh A, et al. Human Umbilical Cord-Derived Mesenchymal Stem Cells in the Treatment of Multiple Sclerosis Patients: Phase I/II Dose-Finding Clinical Study. Cell Transplant. 2024; 33:9636897241233045. https://doi.org/10.1177/09636897241233045
- UofL cardiologist leading clinical trial for high potential new therapy for heart failure — School of Medicine University of Louisville. Louisville.edu. Published 2024-08-06.
- Wang, R., Feng, B., Yao, Q. et al. Human placenta mesenchymal stromal cells alleviate intestinal inflammation and repair intestinal barrier function by activating AMPK-FXR pathway. Commun Biol 2025; 8:830. https://doi.org/10.1038/s42003-025-08261-y
- Barzegar M, Wang Y, Eshaq RS, Yun JW, et al. Human placental mesenchymal stem cells improve stroke outcomes via extracellular vesicles-mediated preservation of cerebral blood flow. EBioMedicine. 2021; 63:103161. https://doi.org/10.1016/j.ebiom.2020.103161
- Fan M, Zhang J, Zhou L, Chen Z, et al. Intra-articular injection of placental mesenchymal stromal cells ameliorates pain and cartilage anabolism/catabolism in knee osteoarthritis. Front Pharmacol. 2022; 13:983850. https://doi.org/10.3389/fphar.2022.983850
- Walkden A. Amniotic Membrane Transplantation in Ophthalmology: An Updated Perspective. Clin Ophthalmol. 2020; 14:2057-2072. https://doi.org/10.2147/OPTH.S208008
- Forbes J, Jackson GR, Knapik DM, et al. The use of amniotic tissue-derived products in orthopedic surgery: A narrative review. Injury. 2024; 55(11):111901. https://doi.org/10.1016/j.injury.2024.111901


