Sie sind hier
Stroke patient recovers after cord blood infusion from StemCyte
 StemCyte is excited to announce the remarkable recovery of a patient who had been paralyzed on one side of his body as the result of a stroke. This patient received an infusion of a matching cord blood unit from the StemCyte public bank. Within a year, he had regained full motor function and images of his brain show that the swelling caused by the stroke had resolved. This case report was published in the peer-reviewed scientific journal CELL TRANSPLANTATION on 23 December 2021.
StemCyte is excited to announce the remarkable recovery of a patient who had been paralyzed on one side of his body as the result of a stroke. This patient received an infusion of a matching cord blood unit from the StemCyte public bank. Within a year, he had regained full motor function and images of his brain show that the swelling caused by the stroke had resolved. This case report was published in the peer-reviewed scientific journal CELL TRANSPLANTATION on 23 December 2021.
Acute stroke is the 2nd leading cause of death and 3rd leading cause of disability worldwide1. More than 15 million people suffer from a stroke annually. Approximately 30% - 35% of these patients die and nearly 75% of survivors sustain permanent disability. In ischemic stroke, a blood vessel in the brain is blocked. The best current treatment for ischemic stroke is to infuse thrombolytic agents to break down blood clots and increase blood flow to the brain. However, this intervention is strongly time-dependent: for every minute that treatment is delayed, 2 million nerve cells die in the patient’s brain2. In the United States, the optimal time window for stroke intervention has been dubbed the “Golden Hour” after onset of stroke.
StemCyte registered a clinical trial NCT02433509 in cooperation with doctors at Taiwan’s Tzu Chi University Hospital that was designed to test the ability of cord blood infusions to help stroke patients. Although the trial was first registered in 2015, it has been difficult to enroll patients because of the requirement that they must find ischemic stroke victims that had missed the window for administration of anti-clotting drugs. The goal of the researchers is to measure the impact of the cord blood infusion alone, with no other stroke intervention in the patient’s system.
In June of 2019, a 46-year-old male patient came to the hospital suffering from a stroke that had begun two hours earlier. This patient had a history of chronic high blood pressure and had been on dialysis for end stage kidney disease since 2011. The doctors realized that he was a candidate for the cord blood study and immediately obtained a baseline MRI image of his brain. The patient gave informed consent to participate in the study, and a request was sent to StemCyte to find a donated cord blood unit that was a match for the patient.
Several clinical trials in multiple countries are currently testing the use of blood stem cells as therapy for stroke3. Cord blood stem cells have several advantages over products that must be manufactured from the blood of the patient or from adult donors. There are the obvious practical advantages that cord blood products are already in storage, without having to find a donor, collect cells from the donor, and screen for diseases. Plus, cord blood products are much less likely to trigger a graft versus host reaction than blood cells from adult donors. Moreover, the authors of the StemCyte study present evidence that cryopreserved cord blood is significantly richer than adult blood in anti-inflammatory proteins that enable cells to communicate and significantly richer in cell growth factors1. Specifically, cord blood has relatively more of the anti-inflammatory cytokine interleukin (IL)-10, as well as the growth factors epidermal growth factor (EGF), vascular endothelial growth factor (VEGF), and granulocyte-macrophage colony-stimulating factor (GM-CSF)1.
Back in June 2019, StemCyte found a cord blood unit that matched the 46-year-old stroke patient at 6 out of 6 HLA types. The cord blood came from a baby that was born in Taiwan in July 2002, 17 years earlier! The cord blood had been processed with a plasma depletion method and had a total mononuclear cell count of 263 million. By the time the unit had been tested, transferred, thawed, and prepared for infusion, it was given to the patient on the 8th day after his stroke. In addition, the patient received four 100mL infusions of mannitol, starting a half hour after the cord blood and every 4 hours thereafter. Mannitol is a diuretic that is frequently given to patients suffering from traumatic brain injury, to lower their intracranial pressure. However, mannitol alone is not a therapy for brain injury because it has not been shown to improve long term outcomes4.
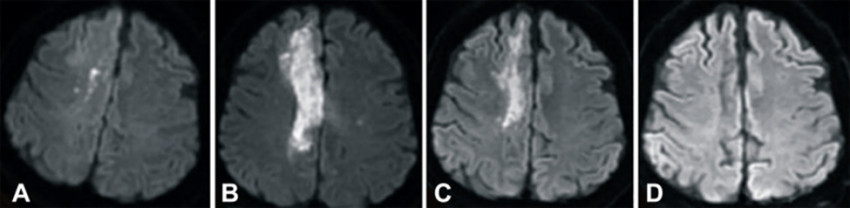
The images below show magnetic resonance images (MRIs) of the stroke patient’s brain that were taken (A) 2 hours after the stroke, (B) 1 day after the umbilical cord blood (UCB) transfusion, (C) 3 months after UCB transfusion, and (D) 6 months after UCB transfusion. These MRI’s were taken with Diffusion-Weighted Imaging (DWI), which is a technique that highlights brain swelling and can accurately detect ischemic stroke within minutes of onset5. The images clearly show that the edema (swelling from excess fluid) in the right lobe of the patient’s brain disappeared within 6 months after the cord blood infusion.
The true test of how well a therapy treats stroke is when a patient regains skills that had been impaired. In this case the patient was experiencing paralysis on the left side of his body (hemiplegia) as a result of the infarction on the right side of his brain. The patient began to regain mobility after the cord blood infusion, and by the third month he could walk with limited assistance. The patient was followed for one year following cord blood therapy. During this time his score on the NIH Stroke Scale (NIHSS) decreased from 9 to 1, the Berg Balance Scale score increased from 0 to 48; and the Barthel index which scores activities of daily living increased from 0 to 90 over the one year follow up. At the end of the observation period, the patient had fully recovered his motor functions and could live independently.
Whereas most stroke patients are left with permanent disabilities, especially if they are not treated within the Golden Hour after stroke, this patient fully recovered after a simple cord blood infusion on the 8th day after his stroke. This could open a new landscape of hope for stroke patients around the world. This case report also demonstrates the potential value of cord blood donations sitting in public banks, even donations from decades ago. Whereas cord blood donations have long been promoted as a life-saving therapy for patients with blood cancers, they might have even broader application as a therapy for stroke patients.
References
- Lee T-K, Lu C-Y, Tsai S-T, Tseng P-H, Lin Y-C, Lin S-Z, Wang JC, Huang C-Y, ChiuT-L. Complete Restoration of Motor Function in Acute Cerebral Stroke Treated with Allogeneic Human Umbilical Cord Blood Monocytes: Preliminary Results of a phase I Clinical Trial. Cell Transplantation 2021; 30:1-7.
- Saver JL, Smith EE, Fonarow GC, Reeves MJ, Zhao X, Olson DWM, Schwamm LH, and on behalf of the GWTG-Stroke Steering Committee and Investigators. The “Golden Hour” and Acute Brain Ischemia. Stroke 2010; 41(7):1431-1439.
- Verter F. Research on Allogeneic Cord Blood for Stroke. Parent's Guide to Cord Blood Newsletter Published 2019-09
- Wang K, Sun M, Jiang H, Cao XP, Zeng J. Mannitol cannot reduce the mortality on acute severe traumatic brain injury (TBI) patients: a meta–analyses and systematic review. Burns & Trauma 2015; 3:8.
- GE Healthcare. MRI of the Brain to Diagnose and Monitor Stroke. Website Last updated 2019-01-11