Jste zde
Parent’s Guide to Duke’s Research and Expanded Access Program of Cord Blood Therapies for Neurodevelopmental Disorders
For 17 years, parents from all over the world have traveled to Duke University Medical Center (Duke hereafter) to seek stem cell therapy for their child that has cerebral palsy, or autism, or one of several other neurodevelopmental disorders. During this time, Duke has enrolled hundreds of children into 19 clinical trials using cells from cord blood or from cord tissue as therapy for neurodevelopmental disorders. In addition to their trials, Duke has also offered off-trial therapy of cord blood stem cell infusions to children with neurodevelopmental disorders.
Prior to 2010, the FDA did not have any regulations on banking or therapies with cord blood1. Once the FDA began to regulate cord blood therapy as a biologic drug, all of Duke’s therapy programs have operated under Investigational New Drug (IND) approvals from the FDA. Since October 2017, the off-trial treatment has been operating in accordance with an FDA-approved Expanded Access Program (EAP) that allows children to receive infusions with their own cord blood or cord blood from a sibling. The parents of these children supply cord blood that they paid to store in private banks when their child was born. The parents have also paid Duke to cover the costs of delivering the off-trial therapy2. Some parents have received partial reimbursement of these costs through the Family Support Program for Pediatric Transplant and Cellular Therapies at Duke, and occasionally from their health insurance plan2,3. There are multiple on-line forums and private discussion groups where thousands of parents are discussing and debating whether or not to try this therapy option4-6.
It turns out that the Duke EAP may be too popular for its own good. Over 17,000 families have joined a waiting list to enroll in the EAP, but only 464 children have been treated over the first 3.5 years of operation7-9. Parents have become so frustrated with the years long waiting list for the EAP that many have given up and gone elsewhere for stem cell therapy, either at legal clinics overseas, or at illegal clinics both in the United States and overseas (where they may not be getting safe stem cell products)10. In spring of 2021, Duke broke this bottleneck by signing a licensing agreement with the private cord blood bank Cryo-Cell, which would enable Cryo-Cell to set up an independent infusion clinic and efficiently provide treatment to over 1500 EAP families per year11-13. Additional terms of the contract will be described below.
Suddenly, a group of vocal opponents have started a campaign against what they perceive as the “commercialization” of Duke’s EAP program. Media reports have accused the researchers of promoting autism treatments (only one of the diagnoses in the EAP) with an “unproven” therapy. Parents may be confused by conflicting stories that either extoll the promise of cord blood therapy or dismiss it as a scam. The purpose of this article is to tackle these questions, with special emphasis on the perspective of parents with special needs children. Let’s dig in.
“ We also had huge gains after Duke...at that point I knew this is something that can be treated. We went from being in fight or flight nearly all the time to being calm and cooperative nearly all the time. I’ll always be grateful for Duke and have no doubt it was a turning point for my son! ” – Pamela F.
The United States FDA has a pathway that allows patients with serious conditions to receive access to therapies that are not yet fully approved for the market. This is referred to by several names, including “Compassionate Use” or “Expanded Access”. When a whole group of patients are covered by the same FDA approval it is often referred to as an Expanded Access Program or EAP.
 The federal laws governing expanded access are published publicly as part of the Code of Federal Regulations (CFR), and the FDA has issued additional Guidance for Industry14,15. Expanded access is designed to give compassionate treatment to patients with conditions that are “life-threatening or have a substantial and long-term impact on day-to-day functioning”. The patient must have a condition for which “there is no comparable or satisfactory alternative therapy”, and where the “potential patient benefit justifies the potential risks”. The FDA approval for expanded access may cover individual patients or groups of patients. During the AIDS crisis in the 1980’s, over 100,000 patients received access to antiretroviral drugs via several EAP approvals16. Thanks to the FDA’s EAP approvals, patients that have serious conditions, who cannot enroll in clinical trials and have no other options, can still get compassionate access to cutting edge therapies.
The federal laws governing expanded access are published publicly as part of the Code of Federal Regulations (CFR), and the FDA has issued additional Guidance for Industry14,15. Expanded access is designed to give compassionate treatment to patients with conditions that are “life-threatening or have a substantial and long-term impact on day-to-day functioning”. The patient must have a condition for which “there is no comparable or satisfactory alternative therapy”, and where the “potential patient benefit justifies the potential risks”. The FDA approval for expanded access may cover individual patients or groups of patients. During the AIDS crisis in the 1980’s, over 100,000 patients received access to antiretroviral drugs via several EAP approvals16. Thanks to the FDA’s EAP approvals, patients that have serious conditions, who cannot enroll in clinical trials and have no other options, can still get compassionate access to cutting edge therapies.
The federal laws as well as internal FDA guidance make it very clear that a drug developer is not allowed to use an EAP as a way to sell a product to consumers without pursuing full FDA approval. In order to be eligible for an EAP, the sponsor must be “actively pursuing marketing approval”. This means they must continue to run clinical trials that will provide the evidence needed for FDA market approval, and they must demonstrate that the EAP will not jeopardize their ability to recruit patients for those trials.
The Duke EAP covers a wide variety of neurodevelopmental conditions, including but not limited to: Hypoxic Ischemic Encephalopathy (HIE), Hydrocephalus, Apraxia, Cerebral Palsy (CP), and Autism Spectrum Disorder (ASD). Both cerebral palsy and autism are well-known to be lifelong conditions for which there is no cure. The CDC estimates that among 8-year-olds in the United States, 1 in 345 have cerebral palsy and 1 in 54 have autism17,18. In the United States the number of people living with these conditions is 0.8 million for cerebral palsy and 3.5 million people on the spectrum13. The target demographic for the Duke EAP is children that have significant impairments in their daily functioning, and are young enough that their brains are still building neural connections. Moreover, families can only enroll in the Duke EAP if they have cord blood stored for the patient or a sibling, and provided that cord blood unit meets the eligibility criteria.
 Numerous studies have shown that developmental disabilities have both direct and indirect costs to families and society. According to the charity Let’s Cure CP, 1 in 3 children with cerebral palsy cannot walk; 1 in 4 cannot feed themselves; and 1 in 4 cannot dress themselves19. In 2003, the average lifetime costs of cerebral palsy were estimated to be $921,00020. The majority of that amount (81%) was from indirect costs such as lost productivity of the patient and caregivers.
Numerous studies have shown that developmental disabilities have both direct and indirect costs to families and society. According to the charity Let’s Cure CP, 1 in 3 children with cerebral palsy cannot walk; 1 in 4 cannot feed themselves; and 1 in 4 cannot dress themselves19. In 2003, the average lifetime costs of cerebral palsy were estimated to be $921,00020. The majority of that amount (81%) was from indirect costs such as lost productivity of the patient and caregivers.
Whereas cerebral palsy is characterized by motor deficits, autism spectrum disorder primarily impacts the development of language and social skills. There is a huge range of skills among people on the spectrum, from those who tend to be labeled “high functioning” (also known as neuro-typical passing) versus people that are non-verbal21. A study in 2014 found that the average lifetime cost of supporting an individual with autism, without intellectual disability, was $1.4 million, driven in childhood by costs for educational services and loss of parental productivity, and in adulthood by residential accommodations and productivity loss22.
This is a very simplified overview of some of the challenges faced by families that might be interested in pursuing the Duke EAP therapy. There are adults on the spectrum that find talk of autism “cures” offensive. Autism advocates are also concerned that some parents who think they are helping their children may have actually fallen for pseudoscience23,24. The scientific evidence that cell therapy holds promise for neurodevelopmental disorders is real (see next section), and most of the participating parents realize that they are pursuing functional “gains”, not “cures”.
The outcomes of previous clinical trials provide the evidence that cord blood infusions can benefit children with neurodevelopmental disorders. In the table below we summarize those trials from Duke University that specifically used unmanipulated cord blood cells to treat neurodevelopmental disorders. Duke is also conducting clinical trials that treat neurodevelopmental disorders with cells from umbilical cord tissue, but those are not listed here because they are not part of the Expanded Access Program.
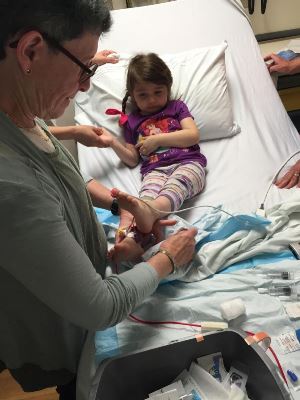 Parents are often confused about the distinction between cells from cord blood versus cells from cord tissue. The stem cells in cord blood are called Hematopoietic (blood-forming) Stem Cells or HSC. The cells from cord tissue are called Mesenchymal Stromal Cells or MSC. The stem cells in cord blood represent a tiny fraction of all the cells in a cord blood unit. The HSCs are the cells that are active when cord blood is used for a stem cell transplant. During a transplant, the patient is pre-treated with lethal doses of chemotherapy and/or radiation, and then rescued with donor cord blood that engrafts, grows a new immune system, and becomes permanent.
Parents are often confused about the distinction between cells from cord blood versus cells from cord tissue. The stem cells in cord blood are called Hematopoietic (blood-forming) Stem Cells or HSC. The cells from cord tissue are called Mesenchymal Stromal Cells or MSC. The stem cells in cord blood represent a tiny fraction of all the cells in a cord blood unit. The HSCs are the cells that are active when cord blood is used for a stem cell transplant. During a transplant, the patient is pre-treated with lethal doses of chemotherapy and/or radiation, and then rescued with donor cord blood that engrafts, grows a new immune system, and becomes permanent.
Cord blood therapy for neurodevelopmental disorders is different from cord blood transplants, because the patient does not go through a preparation regimen and the infused cord blood is not expected to take over their immune system. When treating neurodevelopmental disorders, cord blood cells are used as a “living drug” or biologic therapy. Researchers are actively studying which of the cells in cord blood produce the benefit of the therapy and how long they stay in the body.
The safety of cord blood infusions, either with the child’s own autologous cells or from donors with full or partial HLA-match, has been conclusively proven. Over 40,000 people worldwide have received cord blood transplants for malignant or genetic conditions25. For the diagnosis of cerebral palsy alone, there have been 77 stem cell clinical trials worldwide, treating over 2427 children, including over 900 treated with cord blood (these numbers have been adjusted to incorporate the Duke EAP patients)9,26. Cord blood infusions are very safe when they are sourced and handled by properly trained medical centers. The question for parents is whether they are worth trying.
Whenever any new type of therapy is developed, the initial trials are only intended to establish safety and feasibility. There may be anecdotal reports of individual patients that had big gains, but rigorous research requires a follow up trial to compare those gains against a placebo group. Moreover, since patients often improve from the “placebo effect” alone, the two groups should be blinded so that neither the patients nor their doctors know who is getting therapy versus placebo. This is called a “randomized, blinded, controlled trial”.
Parents of children with developmental disabilities usually are not willing to enroll in a traditional trial against a placebo. They want to make sure that their child has a chance at the therapy. In order to enroll children with conditions like cerebral palsy or autism, the major research centers offer “cross-over” trials. In a cross-over trial, the patients on the treatment arm of the study get cell therapy at the beginning and then a placebo infusion at the end, whereas the control group gets the placebo first and then cell therapy at the end. This guarantees that all the children participating in the trial receive cell therapy. But for the researchers this makes the ability to measure a benefit from cell therapy even more challenging. First, they have to compare improved functional scores against a moving baseline, because children’s scores will naturally improve with age without therapy. On top of that, they have to detect any performance edge from cell therapy that appears during the 6 to 12 months head start that study participants had before the cross-over. To give an example of the potential pitfalls, a trial that gave cord blood for autism at Sutter Health failed to show any benefit, and this is believed to be because the cross-over was set at only 24 weeks, and also because the dose was too low (16M cells/kg)27.
Diagnosis, CORD BLOOD HSC Trial Name: NCT#, Status | Clinical Trial Outcomes |
Acquired Brain Injuries, there is no IND# or Trial# because at this time the FDA did not require them, Published 2010 | Precursor to current EAP: Autologous cord blood stem cell (HSC) infusions given to 184 children with various brain conditions from 2004 to 2009. Cord blood was supplied by 24 private banks around the world. Procedure shown to be safe and feasible. No systematic reporting of outcomes. |
Hydrocephalus, there is no IND# or Trial# because at this time the FDA did not require them, Completed & Published 2015 | Multiple infusions of autologous cord blood HSC given to 76 infants born with hydrocephalus from 2006 to 2014. In most cases the cord blood collection was planned prior to the baby’s delivery. Multiple infusions were possible because the patients were so small. The procedure was safe and feasible. |
Hypoxic Ischemic Encephalopathy (HIE), BabyBac: NCT00593242, Completed & Published 2014 | Autologous cord blood HSC infusion given to 23 infants born from 2009 to 2012 with moderate to severe HIE. These children also received whole body cooling, and were compared to 82 infants that received cooling alone. (This was not structured as a randomized controlled trial.) Despite the difficulty of collecting cord blood during distressed births, at 1 year the rate of survival, with a Bayley III score in 3 domains over 85%, was 74% on cord blood arm vs 41% without cord blood (p=0.04). |
Hypoxic Ischemic Encephalopathy (HIE), BabyBac II: NCT02612155, Completed & Published 2020 | Autologous cord blood HSC infusion was given to 16 infants born from 2015 to 2019 in a randomized, blinded, placebo-controlled trial that enrolled 37 babies. At 1 year follow up, survival and function were trending better on the cord blood arm. This multi-site trial was closed before full enrollment because most hospitals cannot efficiently collect cord blood from distressed births. |
Cerebral Palsy, CP-AC: NCT01147653, Completed & Published 2017 | Autologous cord blood HSC infusion given to 63 children with spastic CP ages 1-6 years. This was a randomized, blinded, placebo-controlled trial that ran from 2010 to 2016 treating children on two arms that crossed over at 1 year and then were followed out to 2 years from the start. Cord blood was supplied by 16 private banks around the world. The children in the “cord blood” group (receiving cord blood at the 1st infusion) had significantly better than expected improvements in motor function and whole brain connectivity of motor tracks, but only for infused cell doses greater than 20M cells/kg (p=0.02). |
Cerebral Palsy, CP Sibling: NCT02599207, Completed & Published 2021 | Sibling cord blood HSC infusion given to 15 children with spastic CP of median age 3.7 years. Cord blood was supplied by 8 private banks. The purpose of this trial was to demonstrate the safety of cord blood from partially HLA-matched donors. Improvements in motor skills were similar to the CP-AC trial, but there was no control group. |
Cerebral Palsy, AcceNT-CP: NCT03473301, Completed & Abstract Published 2021 | This was a randomized open-label trial in which 91 children with hypertonic CP, median age 3.5 years, were treated on 3 arms and followed for 1 year. The arms: (1) Partially HLA-matched cord blood HSC infusion from an unrelated donor at baseline, (2) off-the-shelf umbilical cord MSC infusions, (3) A “natural history” arm where the participant was watched for 1 year and then given partially HLA-matched cord blood HSC infusion from an unrelated donor. Doses were up to 100M cells/kg for cord blood and 3 doses of 2M cells/kg for MSC. This study ran from 2018 to 2020 and experienced disruption from the COVID-19 pandemic. Preliminary results show a statistically significant (p=0.02) improvement in motor skills of children that received high dose cord blood on arm (1), but no improvement in children who received MSC. |
Autism, ABC: NCT02176317, Completed & Published 2017 | Autologous cord blood HSC infusion given to 25 children with autism of median age 4.6 years, then followed for 1 year. The children were screened to not have known genetic anomalies. Cord blood was supplied by 2 US private banks and 1 public bank. The procedure was safe and feasible. A number of behavioral scores and physiologic measures improved during the study and were evaluated for use in a follow up study. |
Autism, Duke ACT: NCT02847182, Completed & Published 2020. | This was a randomized, blinded, placebo-controlled trial with a cross over after 6 months that enrolled 180 children with autism of mean age 5.5 years. Children were screened to exclude known genetic anomalies. All children received cord blood HSC infusions; the two arms of the study were cord blood at 1st visit or cord blood 6 months later. The cord blood was either the child’s own (autologous) or from a donor that was partially HLA-matched. Everyone was followed for 1 year after cross-over, so the study ran from 2016 to 2019. The cord blood dose for all children was over 25M cells/kg, but children receiving donated cord blood had higher mean doses. This study was flawed because it included more children below IQ 70 than the design intended. The study failed to meet its primary endpoint, which had hoped to see a significant change in the VABS-3 socialization scale for all children that received cord blood at the 1st visit. Instead, for the sub-group of children ages 4-7 with non-verbal IQ over 70, the study did show significant improvements in VABS-3 communication scale (p=0.05), eye tracking (p=0.02), and EEG brain scans (p=0.02). |
Expanded Access Program: NCT03327467, Ongoing, Abstracts published 2019 & 2021 | As of June 2021, 2001 children have been fully screened, 464 have been treated, the ratio of autologous to sibling cord blood was 50%/50% and the fraction of children diagnosed with autism was 60%. |
Boiled down to the most basic conclusions, the clinical trials by Dr. Kurtzberg and Duke University that are listed in the table have been able to find statistically significant improvements in the performance scores of children that received cord blood cell therapy for Hypoxic Ischemic Encephalopathy (HIE), Cerebral Palsy (CP), and Autism Spectrum Disorder (ASD). The clinical trials in the table have been written up in multiple papers in peer-reviewed journals (the publications cited are only the key papers, not a full list)8,9,28-37.
But - and there is a but - the study results also showed that cord blood cell therapy only gave significant gains for certain patients and at certain cell doses. The randomized controlled trial for cerebral palsy showed a dose-response effect, where only the children who received higher doses (over 20M cells/kg) had significant improvements. In the case of autism, the randomized controlled trial only showed significant gains for children with non-verbal IQ over 70. In situations like this, when a trial fails to meet its endpoint for the entire patient group, but demonstrates efficacy for a sub-group, the drug developer will run more studies focused on the sub-group. That is the approach being taken by the research team at Duke.
 Some academics live in a simplified world of black and white, where only therapies with full FDA approvals are “proven”, and everything else is “unproven”. By their reasoning, any therapies that lack full FDA approval, regardless of their promise in controlled clinical trials, fall into the bin of unproven therapies.
Some academics live in a simplified world of black and white, where only therapies with full FDA approvals are “proven”, and everything else is “unproven”. By their reasoning, any therapies that lack full FDA approval, regardless of their promise in controlled clinical trials, fall into the bin of unproven therapies.
The problem with the proven versus unproven world view is that it sets up a “false dichotomy”, in the words of Dr. Chris Centeno38. Clinical physicians that treat patients do not practice medicine that way, they must live with shades of gray and prescribe treatments on the basis of “best available evidence”38. Especially in pediatrics, it is very common for doctors to prescribe therapies outside of their FDA approvals, a practice known as “off-label use”. It has been estimated that at least 1 in 4 children that are hospitalized receive a medication that was prescribed off-label39. The use of technically unproven drugs is common in pediatrics because it is difficult to run extra clinical trials to prove that drugs tested on adults are also effective for children. A Commentary published in the Journal of Pediatrics in 2019 states: “…off label is not synonymous with off evidence. Although drugs are often used off label, there may be sufficient preliminary research about a medical condition and particular drugs to support their use”40.
A vocal group of critics is responsible for multiple blogs and interviews that attack Dr. Kurtzberg’s research and the Duke EAP41-46. Two themes recur. First, the authors characterize all the Duke clinical trials as having been complete failures, ignoring the positive results seen for sub-groups of patients. Second, the word “unproven” recurs over and over again. Writers for the mass media just lap up this “unproven” accusation. Anything that sensationalizes a topic, like claiming that a big business is being set up to offer parents an “unproven” autism cure, acts like click bait to draw in readers46.
Cryo-Cell International (publicly traded on Nasdaq with the stock symbol CCEL) is the company that has signed an exclusive license with Duke to further develop and commercialize cord blood and cord tissue therapies, and to set up independent infusion clinics to treat patients approved under the Duke EAP. In the summer of 2021, Cryo-Cell created a slide deck for investors with some aspirational numbers about their planned infusion clinic(s)13. The slide deck stated that the first infusion clinic would charge $15K per patient, and should be able to generate revenues of $24M per year. More than anything else, it was the announcement that the fee per patient would be $15K that has set off a storm of controversy45,46, even though Duke has been charging that amount for off-trial therapy for several years.
Legal questions have been raised by the critics. Some professionals in the cell therapy field are under the impression that an EAP can only charge participants for the cost to manufacture the therapy, and not for the cost to deliver the therapy. They are mistaken, and should read the guidelines, which are publicly available, for charging under Expanded Access47,48. The sponsor of the EAP is allowed to charge for the cost of delivering the therapy and for administrative expenses, the sponsor is allowed to hire a third party (like Cryo-Cell) to run the program, and the third party is allowed to make a profit48. If the sponsor will charge patients for access, the FDA requires the sponsor to submit a cost recovery plan, and that plan must be reviewed by an independent certified public accountant48. Thus, what Duke and Cryo-Cell are doing is completely legal. In the case of the off-trial therapies that have been offered by Duke and will be given by the Cryo-Cell infusion clinic(s), the cost that is being recovered is the medical care to safely prepare and deliver the cord blood infusion, and not the cost of the cord blood itself.
 It may seem shocking that Duke Health needs to charge parents for the costs of receiving off-trial therapy at their clinic. However, the off-trial therapy delivered under the Duke EAP is not covered by research grant funding and, because it is not FDA approved yet, is not reimbursed under most health insurance plans. Hence the cost of treating large numbers of patients under the Duke EAP would become a burden on the medical center if charges could not be passed on somehow. Duke’s Vice President of Finance has pointed out that there is a big difference between having lots of money versus being able to spend it at will. In his words, “This is essentially because the vast majority of our existing resources are restricted as to use, and because there are always many ambitions across Duke to do new and exciting things”49.
It may seem shocking that Duke Health needs to charge parents for the costs of receiving off-trial therapy at their clinic. However, the off-trial therapy delivered under the Duke EAP is not covered by research grant funding and, because it is not FDA approved yet, is not reimbursed under most health insurance plans. Hence the cost of treating large numbers of patients under the Duke EAP would become a burden on the medical center if charges could not be passed on somehow. Duke’s Vice President of Finance has pointed out that there is a big difference between having lots of money versus being able to spend it at will. In his words, “This is essentially because the vast majority of our existing resources are restricted as to use, and because there are always many ambitions across Duke to do new and exciting things”49.
The contract between Duke and Cryo-Cell is an example of an academic institution that has developed a promising therapy and then licensed it to a biotech company for commercialization. It is very challenging for a university to achieve a successful technology transfer to the commercial marketplace without outside investment50. The Cryo-Cell partnership does more than just set up a clinic to process patients on the EAP waiting list; it also promises Cryo-Cell support for late phase trials that hopefully will lead to market approval of therapies for hypoxic ischemic encephalopathy (HIE), cerebral palsy, and autism spectrum disorder51.
At the end of the day, the ethical aspects of the Duke EAP need to be separated from the financial aspects. An ethicist can make a strong case that it is unethical to charge families for compassionate treatment of a child that has disabilities which impact daily life. But the financial reality is that Duke does not have the resources to scale up their EAP. This means that the Duke EAP will never get bigger unless another entity, such as Cryo-Cell, licenses the technology and offers it commercially.
The saddest part of this story is that parents of children with severe cerebral palsy or autism do not have viable and affordable alternatives. If parents want to try cell therapy, the Duke EAP is the only legal avenue in the United States. The other option that is very popular with this parent community is the Panama Stem Cell Institute, which is not subject to FDA oversight, and where the cost of therapy is typically $20K, or 33% higher than the proposed fee of the Cryo-Cell infusion clinic13,52. Parents are already facing average lifetime costs near a million dollars to raise a child with significant disabilities20,22. Seen against the alternatives, it is not surprising that parents are willing to raise the money to participate in the Duke EAP. Also, the parents participating in the Duke EAP have already chosen to invest in potential cord blood therapy by banking the cord blood of their child or a sibling at birth.
One blogger has called for the FDA to freeze the Duke EAP45. That is not likely to happen, because the FDA approves 99% of EAP requests53. But what would parents do if the FDA shut down the Duke EAP? The answer is that parents would continue to seek stem cell therapy elsewhere. In doing so, they may spend more money and put their child at risk. With stem cell therapy for neurodevelopmental disorders, the proverbial genie is out of the bottle. There are quite a few testimonials circulating of children that had remarkable improvements after stem cell therapy. These stories are being shared in Facebook groups4-6 and some have been published in the newsletter of this Foundation54-65. These testimonials are not just a pseudoscience marketing campaign, because they are not all coming from one source or describing treatment at one center. The testimonials are motivating parents to try it, and parents are not going to stop just because critics think they are too vulnerable to understand what they are doing.
At the Parent’s Guide to Cord Blood Foundation, we believe in treating parents like adults. We endeavor to empower them with accurate information and then let them decide for themselves what is in the best interests of their family. We do not steer parents to specific clinics, except to warn parents away from treatments that are clearly dangerous. Please remember that the website of Parent’s Guide to Cord Blood Foundation is not a substitute for medical advice from a physician.
Parent’s Guide to Cord Blood Foundation is incorporated as a 501c3 charity. As such, our annual IRS filings are a matter of public record. Our last complete fiscal year was the calendar year 2020, and during that year we reported revenues of $193K. This included an unrestricted grant of $15K from Cryo-Cell International. Our Foundation has received comparable donations from Cryo-Cell every year since we incorporated. The Foundation has never received any donation from Duke University. Our newsletter is published monthly and never runs articles for pay. We have frequently published articles about the work of Dr. Joanne Kurtzberg at Duke University. She is the world’s most prolific and most famous researcher in the field of cord blood therapy, so any newsletter that covers this industry is bound to report closely on her work.
- Rao M. Cord Blood and the FDA. Parent's Guide to Cord Blood Foundation Newsletter Published 2015-01
- Verter F, Kurtzberg J, Dunay L, Pelz A. Accessing the Value of Cord Blood Therapies. Parent's Guide to Cord Blood Foundation Newsletter Published 2014-02
- Duke. Pediatric Transplant and Cellular Therapy Family Support Program. https://dukepbmtfamilysupport.org/services Accessed 2021-11-01
- Facebook. Stem Cell Therapy for Autism - Duke Univ Clinical Trials & Expanded Access. Private Group (4901 members) Accessed 2021-11-01
- Facebook. Stem Cell Therapy for Autism. Private Group (13,530 members) Accessed 2021-11-01
- Facebook. Stem Cells for HIE. Private Group (4238 members) Accessed 2021-11-01
- Kurtzberg J. Oral presentation at Phacilitate Advanced Therapies Meeting 2019, Stated 2019-01-24
- McLaughlin CA, West T, Hollowell R, Skergan NN, Baker J, Donner H, Cash J, Hoyle K, Crane S, Waters‐Pick B, Hawkins T, Page K, Prasad VK, Sun J, Kurtzberg J. Expanded Access Protocol of Umbilical Cord Blood Infusion for Children with Neurological Conditions. Stem Cells Translational Medicine 2019; 8(Suppl):S4-S5.
- McLaughlin C, West T, Hollowell R, Skergan N, Giguere P, Vinesett R, Arbuckle E, Cash J, Hoyle K, Crane S, Moore L, Waters‐Pick B, Hawkins T, Prasad V, Sun J, Kurtzberg J. Expanded Access Protocol of Umbilical Cord Blood Infusion for Children with Neurological Conditions: An Update. Stem Cells Translational Medicine 2021; 10(Suppl):S7-S8.
- Hartnett KP, Powell KM, Rankin D, et al. Investigation of Bacterial Infections Among Patients Treated With Umbilical Cord Blood–Derived Products Marketed as Stem Cell Therapies. JAMA Network Open 2021; 4(10):e2128615.
- Cryo-Cell. Cord Blood Banking Leader Cryo-Cell International Enters Into An Exclusive License Agreement with Duke University. GlobeNewsWire Released 2021-02-26
- Cryo-Cell. Cryo-Cell granted exclusive rights to Duke therapies. Parent's Guide to Cord Blood Foundation Newsletter Published 2021-05
- Cryo-Cell. 2021. Our Pluripotent Year. Investor slide deck Published 2021-08-18
- Code of Federal Regulations. Expanded Access to Investigational Drugs for Treatment Use. 21CFR312 subpart I Published 2009-08-13
- FDA. Expanded Access to Investigational Drugs for Treatment Use - Questions and Answers. Guidance for Industry Published 2016-06
- Amorosa A, Tebas P. Is It Time to Rethink the Expanded-Access Programs for HIV Infection? J. Infectious Diseases 2007; 196:974–977.
- CDC. Data and Statistics for Cerebral Palsy. Web page Last updated 2020-12-31
- CDC. Data & Statistics on Autism Spectrum Disorder. Web page Last updated 2020-09-25
- Let’s Cure CP. Facts about cerebral palsy Accessed 2021-11-01
- CDC. Economic costs associated with mental retardation, cerebral palsy, hearing loss, and vision impairment--United States, 2003. Morbidity and Mortality Weekly Report 2004; 53(3):57-9.
- Gardiner F. The Problems with Functioning Labels. Thinking Person's Guide to Autism Blog Published 2018-03
- Buescher AVS, Cidav Z, Knapp M, et al. Costs of Autism Spectrum Disorders in the United Kingdom and the United States. JAMA Pediatrics 2014; 168(8):721-728.
- Autistics for Autistics (A4A). Autism Pseudoscience: A Physician’s Guide Accessed 2021-11-01
- Thinking Person’s Guide to Autism. Mission Accessed 2021-11-01
- Ballen K. Update on umbilical cord blood transplantation. F1000Research 2017; 6:1556
- Paton MCB, Finch-Edmondson M, Fahey MC, London J, Badawi N, Novak I. Fifteen years of human research using stem cells for cerebral palsy: A review of the research landscape. J. Paediatrics Child Health 2021; 57(2):295-296.
- Chez M, Lepage C, Parise C, Dang‐Chu A, Hankins A, Carroll M. Safety and Observations from a Placebo‐Controlled, Crossover Study to Assess Use of Autologous Umbilical Cord Blood Stem Cells to Improve Symptoms in Children with Autism. Stem Cells Translational Medicine 2018; 7(4):333-341
- Sun J, Allison J, McLaughlin C, Sledge L, Waters-Pick B, Wease S, Kurtzberg J. Differences in quality between privately and publicly banked umbilical cord blood units: a pilot study of autologous cord blood infusion in children with acquired neurologic disorders. Transfusion 2010; 50(9):1980-1987.
- Sun JM, Grant GA, McLaughlin C, Allison J, Fitzgerald A, Waters-Pick B, Kurtzberg J. Repeated autologous umbilical cord blood infusions are feasible and had no acute safety issues in young babies with congenital hydrocephalus. Pediatric Research 2015; 78:712–716.
- Cotten CM, Murtha AP, Goldberg RN, Grotegut CA, Smith PB, Goldstein RF, Fisher KA, Gustafson KE, Waters-Pick B, Swamy GK, Rattray B, Tan S, Kurtzberg J. Feasibility of Autologous Cord Blood Cells for Infants with Hypoxic-Ischemic Encephalopathy. Pediatrics 2014; 164(5):973-979.e1
- Cotten CM, Fisher KA, Malcolm W, Gustafson K, Kurtzberg J. Phase II Clinical Trial of Autologous Cord Blood Cells for Neonates with Hypoxic-Ischemic Encephalopathy. Pediatric Academic Societies Abstract 2020
- Sun JM, Song AW, Case LE, Mikati MA, Gustafson KE, Simmons R, Goldstein R, Petry J, McLaughlin C, Waters‐Pick B, Chen LW, Wease S, Blackwell B, Worley G, Troy J, & Kurtzberg J. Effect of Autologous Cord Blood Infusion on Motor Function and Brain Connectivity in Young Children with Cerebral Palsy: A Randomized, Placebo‐Controlled Trial. Stem Cells Translational Medicine, 2017; 6(12):2071-2078.
- Sun JM, Case LE, Mikati MA, Jasien JM, McLaughlin C, Waters‐Pick B, Worley G, Troy J, Kurtzberg J. Sibling umbilical cord blood infusion is safe in young children with cerebral palsy. Stem Cells Translational Medicine 2021; 10(9):1258–1265.
- Sun JM, Case LE, McLaughlin C, Skergan N, Jasien J, Mikati M, Troy J, Kurtzberg J. Umbilical Cord Blood and Umbilical Cord Tissue Mesenchymal Stromal Cells in Children with Cerebral Palsy: A Randomized Trial. Stem Cells Translational Medicine 2021; 10(Suppl):S6.
- Dawson G, Sun JM, Davlantis K, Murias M, Franz L, Troy J, Simmons R, Sabatos-DeVito M, Durham R, Kurtzberg J. Autologous Cord Blood Infusions Are Safe and Feasible in Young Children with Autism Spectrum Disorder: Results of a Single‐Center Phase I Open‐Label Trial. Stem Cells Translational Medicine 2017; 6(5):1332-1339
- Dawson G, Sun JM, Baker J, ... Waters-Pick B, Troy J, Kurtzberg J. A Phase II Randomized Clinical Trial of the Safety and Efficacy of Intravenous Umbilical Cord Blood Infusion for Treatment of Children with Autism Spectrum Disorder. Pediatrics 2020; 222:164-173.E5.
- Kurtzberg J. Results from the Duke ACT Study of Cord Blood for Autism: Highlights for Parents. Parent's Guide to Cord Blood Foundation Newsletter Published 2020-07
- Centeno C. The Passion of Paul Knoepfler - A Review of The Niche Blog. Regenexx Blog Published 2020-02-19
- Yackey K, Stukus K, Cohen D, Kline D, Zhao S, Stanley R. Off-label Medication Prescribing Patterns in Pediatrics: An Update. Hospital Pediatrics 2019, 9(3):186-193.
- Yackey K, Stanley R. Off-Label Prescribing in Children Remains High: A Call for Prioritized Research. Pediatrics 2019; 144(4):e20191571.
- Knoepfler P. Cord blood for cerebral palsy: mostly discouraging new trial data. The Niche Published 2017-10-31
- Knoepfler P. Duke phase II trial: no benefit of cord blood for autism. The Niche Published 2020-05-20
- King AB. Noncompliant - a podcast about neurodiversity and human rights. Podcast Published 2021-05-19
- Knoepfler P. Duke & Cryo-Cell plan big stem cell clinic for kids for unproven infusions. The Niche Published 2021-06-09
- Knoepfler P. FDA should freeze Duke EAP & probe $58 million Cryo-Cell deal linked to it. The Niche Published 2021-08-19
- Merlan A. A Controversial Autism Treatment Is About to Become a Very Big Business. VICE Published 2021-10-06
- Code of Federal Regulations. Charging for investigational drugs under an IND. 21CFR312.8 Updated 2020-04-01
- FDA. Charging for Investigational Drugs Under an IND - Questions and Answers. Guidance for Industry Published 2016-06
- Working@Duke An Inside Look at Duke's Finances. Duke TODAY Published 2019-08-28
- Godfrey PC, Allen GN, Benson D. The biotech living and the walking dead. Nature Biotechnology 2020; 38:132–141.
- Cryo-Cell. Cord Blood Banking Leader Cryo-Cell Announces Its Intent to Advance Clinical Trials. GlobeNewsWire Released 2021-10-27
- Verter F. Stem Cell Therapy for Autism at the Panama Stem Cell Institute. Parent's Guide to Cord Blood Foundation Newsletter Published 2019-05
- Moch KI. Ethical Crossroads: Expanded Access, Patient Advocacy, and the #SaveJosh Social Media Campaign. MA@PoC 2017; 1(1):e119-e130.
- Hemafund. Maksim’s Cord Blood Therapy for Autism. Parent's Guide to Cord Blood Foundation Newsletter Published 2020-03
- Guezamburu M. A Chance for Facu. Parent's Guide to Cord Blood Foundation Newsletter Published 2019-10
- Lovie & Serge. Levi's Story: Cord Blood Research for Autism. Parent's Guide to Cord Blood Foundation Newsletter Published 2019-05
- Alisa & Mayo. Ashton’s Cord Blood Story. Parent's Guide to Cord Blood Foundation Newsletter Published 2019-04
- Victoria. Nerea's Story. Parent's Guide to Cord Blood Foundation Newsletter Published 2019-03
- Parents of Asia. Asia’s Cord Blood Story. Parent's Guide to Cord Blood Foundation Newsletter Published 2019-01
- Shatirishvili G. Nicoloz Story: Autism treatment with intrathecal injection of autologous cord blood. Parent's Guide to Cord Blood Foundation Newsletter Published 2018-08
- Dey Singha A. Apratim gets cord blood therapy for autism. Parent's Guide to Cord Blood Foundation Newsletter Published 2018-04
- Druzin R. Stem Cells Work Wonders on Baby. Parent's Guide to Cord Blood Foundation Newsletter Published 2017-01
- Americord. After Treatment with her Own Cord Blood, Toddler is Able to Speak. Parent's Guide to Cord Blood Foundation Newsletter Published 2016-01
- Miller N. First US patient treated in South Korea for Cerebral Palsy. Parent's Guide to Cord Blood Foundation Newsletter Published 2014-12
- Martini I. Adriana's cord blood therapy for cerebral palsy. Parent's Guide to Cord Blood Foundation Newsletter Published 2014-09


