Jste zde
Multiple Doses with Americord's 5-Chamber Storage Bag
Traditionally, the standard receptacle for freezing cord blood has been a blood bag that had two compartments, each holding 80% and 20% of the total. The photo on the left shows a traditional 80/20 bag manufactured by Pall that is used by a number of public cord blood banks. The 2-chamber bag was acceptable when the primary application of cord blood stem cells was for cord blood transplants. Transplants call for high volumes of stem cells, so that, except for the smallest patients, it was desirable to use the entire stored unit at once. The 80/20 bag allowed for a maximum of two doses.
Since 2005, cord blood has been used for regenerative medicine therapies of conditions like cerebral palsy1, other brain injuries at birth2, and more recently autism spectrum disorder3. Nowadays, the overwhelming majority of parents that consider using their privately stored cord blood for therapy are seeking a regenerative medicine therapy. It has become desirable to be able to give the patient multiple doses of cord blood stem cells.
Scientific research to improve stem cell transplants is also pushing cord blood banks towards storage with the option for multiple doses. There are now several competing methods to “expand” the stem cell count of a cord blood unit prior to therapy4. This makes it possible to give a cord blood transplant with a smaller starting stem cell count, or to give multiple therapies from a single cord blood unit.
Americord Registry, a fast-growing family bank located in New York, offers consumers storage of cord blood, cord tissue, and placental tissue. Americord is committed to keeping up with medical science to bring clients the most beneficial products for their families. As the technology surrounding cord blood banking continues to improve, we continue to innovate to find new ways to support the ongoing possible treatments.
As a result, we have switched our freezing bag to a five-compartment bag manufactured by Pall. In the Pall 5-chamber bag, visible in the photo on the left, each compartment holds 20% of the total volume. Parents storing cord blood today with Americord have the option in the future to use individual compartments - meaning up to five doses – for cord blood stem cell therapies. The option of multiple therapies from a single cord blood collection is now in reach for our clients.
Five-chamber bags can offer the opportunity for multiple infusions. The first regenerative medicine therapies to receive market approval in multiple countries were based on clinical trials that employed multiple infusions5.
When Duke University published their first report on the use of cord blood to treat cerebral palsy, 184 children received 198 infusions of autologous cord blood1. In other words, only 14 of the children were able to receive a repeat infusion. Americord’s use of a five-chamber bag for umbilical cord blood storage would change this paradigm, allowing treating physicians the flexibility to offer multiple infusions.
Expanded cord blood is being used in clinical trials to increase the cell dose for cord blood transplants. The higher the number of stem cells, the faster the patient will experience engraftment to replenish their immune system. As a result, the patients recover sooner when compared to conventional methods. Studies have reported successful results with expanded cord blood for hematologic malignancies6, for inherited metabolic disorders7, and a recruiting trial is testing expanded cord blood on multiple myeloma8.
Given the mounting and promising results of clinical trials, we believe that storing cord blood in a multiple chamber bag is a necessary update. This can only benefit those who elect to store their cord blood by providing options for their family’s medical treatments in the future. Americord is committed to providing products that improve lives, and continuing to seek options that are in the best interest of our clients and their families.
References
- Sun J, Allison J, McLaughlin C, Sledge L, Waters‐Pick B, Wease S, Kurtzberg J. Differences in quality between privately and publicly banked umbilical cord blood units: a pilot study of autologous cord blood infusion in children with acquired neurologic disorders. Transfusion 2010; 50(9):1980-1987.
- Cotten CM, Murtha AP, Goldberg RN, Grotegut CA, Smith PB, Goldstein RF, Fisher KA, Gustafson KE, Waters-Pick B, Swamy GK, Rattray B, Tan S, Kurtzberg J. Feasibility of Autologous Cord Blood Cells for Infants with Hypoxic-Ischemic Encephalopathy. Pediatrics 2014; 164(5):973-979.e1
- Dawson G, Sun JM, Davlantis K, Murias M, Franz L, Troy J, Simmons R, Sabatos-DeVito M, Durham R, Kurtzberg J. Autologous Cord Blood Infusions Are Safe and Feasible in Young Children with Autism Spectrum Disorder: Results of a Single‐Center Phase I Open‐Label Trial. Stem Cells Translational Medicine 2017; 6(5):1332-1339.
- Verter F, Bersenev A., Silva Couto P. Clinical Trials of Expanded Cord Blood. May 2018; Parent's Guide to Cord Blood Newsletter
- Galipeau J & Sensébé L. Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell 2018; 22(6):824-833.
- Horwitz ME, Chao NJ, Rizzieri DA, Long GD, Sullivan KM, Gasparetto C, Chute JP, Morris A, McDonald C, Waters-Pick B, Stiff P, Wease S, Peled A, Snyder D, Galamidi Cohen E, Shoham H, Landau E, Friend E, Peleg I, Aschengrau D, Yackoubov D, Kurtzberg J, Peled T. Umbilical cord blood expansion with nicotinamide provides long-term multilineage engraftment. J. Clinical Investigation 2014; 124(7):3121–3128.
- Orchard PJ, Raffel GD, Condon CEH, Monaghan CA, Braun JA, Shanley R, Lund TC, Gupta A, Boitano AE, Cooke MP, Davis Jr. JC, Wagner JE. Robust Engraftment with Mgta-456, a CD34+ Expanded Cell Therapy Product in Patients with Inherited Metabolic Disorders (IMD): Preliminary Phase 2 Trial Results. Biol. Blood Marrow Transplantation 2019; 25(3):S91-S92
- ClinicalTrials.gov NCT03441958



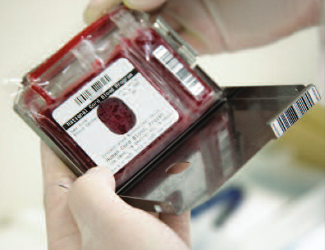 Why a 5-Chamber Storage Bag?
Why a 5-Chamber Storage Bag?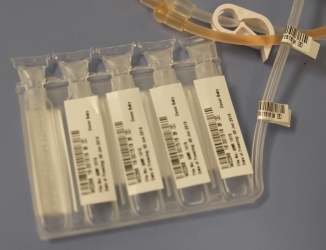 Americord’s 5-Chamber Storage Bag
Americord’s 5-Chamber Storage Bag