現在地
DEHP Regulations Impact Cord Blood Storage
Studies have shown that once cord blood stem cells are cryogenically preserved, they will remain viable for clinical use for decades1,2. Cord blood banks are a legacy resource. In the case of family banks, cord blood that was stored at the birth of a child may be used later when that child has become an adult3. In the case of public cord blood banks, inventory collected from genetically diverse donors can be used to transplant patients from underserved groups decades later, and this potential is enhanced by the development of cell expansion protocols4-6.
Nonetheless, recent shifts in the regulatory landscape affecting cell therapy manufacturing can have a significant impact on the ability to deliver cord blood therapies internationally. One of the biggest concerns is the introduction of environmental legislation prohibiting the use of DEHP in blood collection and storage bags.
DEHP is an acronym for Di(2-ethylhexyl) phthalate. It is a plasticizer, which is a class of chemicals that are added to plastic products to make them more flexible. Phthalates like DEHP have been used in blood bags and other medical devices for over 50 years; DEHP in particular has superior performance at protecting red blood cells7. However, a growing body of evidence has raised concerns about the use of DEHP and other phthalates in medical devices. There are two problems. The first problem is that DEHP is what bioengineers call a “leachable”; small quantities of DEHP leach into any fluid held in the plastic container under normal operating conditions8. The second, and more serious, problem is that studies have shown that DEHP has many toxic properties. Exposure to DEHP can cause disruption to reproduction, neonatal development, endocrine function, and poses carcinogenic risk9,10. Environmental exposure to DEHP has been increasing around the world, but the long-term impact of DEHP exposure in humans, and the role of exposure during medical treatment, is still under study11.

Figure 1: Blood bags participating in a quality study conducted by Canadian Blood Services7.
The European Union (EU) has issued a ban on DEHP in medical devices that will take effect in 2030. The momentum behind this ban has been building for some time. The active legislation is a 2006 EU law called REACH (EC No 1907/2006: regulation concerning the Registration, Evaluation, Authorization, and restriction of CHemicals)12,13. This law governs the production and use of chemicals that impact human health and the environment. REACH has classified DEHP as a substance of very high concern (SVHC) since 201113. Starting in 2019, the EU began restricting DEHP in all plasticized materials, but medical devices were placed in an exempted category (Annex XIV) which gave them until 27 May 2025 to phase out DEHP13,14. Finally, in 2023 EU Regulation No. 2023/2482 amended REACH to extend the sunset date for DEHP in medical devices by five years, until 1 July 203015. Medical device manufacturers who feel they must continue using DEHP in their products beyond the sunset date are required to submit an application for authorization no later than 1 January 202915.
We live in a time when the practice of medicine is globally interconnected; medical devices and therapies often must cross borders in order to reach patients. Any disruption in the supply chain of manufacturing therapies or delivering therapies will impact patient outcomes. In medical fields such as hematopoietic stem cell transplantation and cellular therapy, cross-border cooperation is crucial. A patient in one country may depend on a life-saving therapy that is derived from a cord blood unit that was cryopreserved years earlier in a biobank located in another country4.
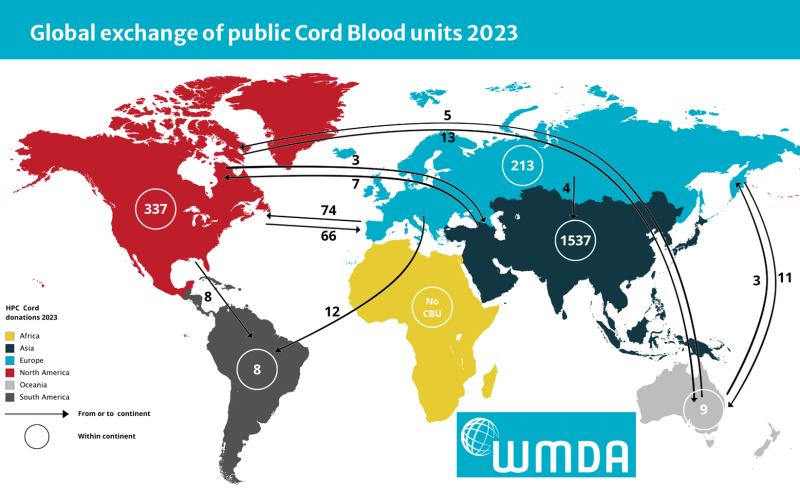
Figure 2: World Marrow Donor Association (WMDA) data on the exchange of cord blood units between continents during 2023
For biobanks in general and cord blood banks specifically, the REACH legislation poses challenges both going forward and looking backward. Going forward, the first challenge is that blood banks and biobanks that are based within the EU have until 2030 to identify and validate acceptable alternatives to DEHP and other banned phthalates for the manufacture of blood bags and associated medical devices like IV tubing, etc. Secondly, the EU regulations will impose constraints on manufacturers in other countries that want to ship products into the EU. Looking backward, biobanks must obtain exemptions to authorize the release of specimens from their pre-existing inventory of cryopreserved cell therapies that are held in bags containing DEHP. It is not feasible to repackage these cryopreserved specimens in new containers. The life-saving potential of these cell therapies should override the DEHP exposure risks posed by their containers. To give an example, evidence-based findings indicate that the survival benefits of medical interventions in the neonatal intensive care unit (NICU) outweigh the neurological risks associated with DEHP exposure in very low birth weight infants19.
The keyword “biobank” is not referenced anywhere within the consolidated corpus of the REACH legislation currently in force, despite its length spanning several hundred pages and its history extending back to 200613.
Meanwhile, the DEHP ban in the EU has inspired a surge of recent laws banning DEHP in individual US states. A thorough review of this legislative patchwork was published in July 2025 by Frontier & Van’t Hof18. California set the precedent, with a law enacted in 2024 that originally proposed to ban DEHP in all medical devices after 1 January 202617,18. Fortunately, stakeholders were able to convince the legislature that this deadline was unrealistic, and the final law set the sunset date to 1 January 2030. The final California law also has an amendment which excludes human blood collection and storage bags, and apheresis and cell therapy blood kits and bags, including integral tubing17,18. Subsequently, a few more states have proposed bans on DEHP (see Table 1 of Frontier & Van’t Hof)18. To date, the enacted state laws have all followed California’s precedent and created carve outs for blood banking18. None of the state laws acknowledge the existence of biobanking. It would be a mistake to be over-confident that US state laws will always follow well-established medical precedents. Politicians in some states have recently decided to write their own state-level cell therapy regulations, with results that are not always rational20,21.
The medical community is under increasing threat from well-meaning politicians who enact laws that are strictly forward-looking, and who seem to have no awareness that life-saving therapies also rely on reaching backward to retrieve cryopreserved specimens in biobanks. It is imperative that stakeholders in biobanking join forces and make their voices heard in the corridors where these regulations are debated and enacted. Biobanks need to prepare and submit applications to the competent EU authorities, before the deadline of 1 January 2029, to request REACH exemptions for archived therapies that are cryopreserved in bags containing DEHP.
References
- Broxmeyer H, Luchsinger L, Weinberg R, Jimenez A, Frenet EM, van't Hof W, Capitano M, Hillyer C, Kaplan M, Cooper S, Ropa J, Abstract 16: Insights into Highly Engraftable Hematopoietic Cells from 27-Year Cryopreserved Umbilical Cord Blood. Stem Cells Translational Medicine. 2023; 12(s1):S18.
- Liedtke S, Többen S, Gressmann H, Meyer A, Verde PE, Gluckman E, Kogler G. Long-Term Stability of Cord Blood Units After 29 Years of Cryopreservation: Follow-Up Data From the José Carreras Cord Blood Bank. Stem Cells Translational Medicine. 2024; 13(1):30-42.
- Verter F. Cured by his own cord blood 19 years later. Parent's Guide to Cord Blood Foundation Newsletter. Published 2023-10
- Elwood N. Why we do what we do. LinkedIN. Published 2018-11-06
- Verter F. ExCellThera Can Improve Patient Access to Cord Blood Transplants. Parent's Guide to Cord Blood Foundation Newsletter Published 2022-09
- Chan A, Seymour C. UM171 Cell Therapy Earns Positive CHMP Opinion (EMA) for Hematological Malignancies. OncLive News. Published 2025-06-20
- Walsh G. Searching for safer red blood cell bags for pediatric recipients. Canadian Blood Services. Published 2016-08-10
- Ding W, et al. Best Practices Guide for Evaluating Leachables Risk from Polymeric Single-Use Systems Used in Biopharmaceutical Manufacturing. BioPhorum Operations Group. Published 2021-07
- Latini G, De Felice C, Verrotti A. Plasticizers, infant nutrition and reproductive health. Reproductive Toxicology. 2004; 19(1):27-33.
- Rowdhwal SSS, Chen J. Toxic Effects of Di-2-ethylhexyl Phthalate: An Overview. BioMed Res. Intnl. 2018; 2018:1750368.
- Qu J, Xia W, Qian X, Wu Y, Li J, Wen S, Xu S. Geographic distribution and time trend of human exposure of Di(2-ethylhexyl) phthalate among different age groups based on global biomonitoring data. Chemosphere. 2022; 287(2):132115.
- European Chemicals Agency. Understanding REACH. Legislation.
- Consolidated text: Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), establishing a European Chemicals Agency, amending Directive 1999/45/EC and repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC (Text with EEA relevance). EUR-Lex. Last updated 2025-04-02 [2019]
- European Chemicals Agency. Endocrine disrupting properties to be added for four phthalates in the Authorisation List. News. Published 2019-07-10
- Official Journal of the European Union. COMMISSION REGULATION (EU) 2023/2482 amending Regulation (EC) No 1907/2006 of the European Parliament and of the Council as regards the substance bis(2-ethylhexyl) phthalate (DEHP) in medical devices. EU2023/2482. Published 2023-11-13
- Killela P, Herrity K, Frontier L, Horton R, Kurtzberg J, Van’t Hof W. Mitigation of supply chain challenges in cell therapy manufacturing: perspectives from the cord blood alliance. Stem Cells Transl. Med. 2024; 13(9):843–847.
- Frontier LR. The Moral Duty to Protect the Supply of Quality Health Care. ISCT Telegraft. Published 2024-08-15
- Frontier L. & Van’t Hof W. Navigating non-DEHP legislation while protecting cryopreserved cord blood inventories during regulatory transitions. Cell & Gene Therapy Insights. 2025; 11(5):717-725.
- Stroustrup A, Bragg JB, Andra SA, Curtin PC, Spear EA, Sison DB, Just AC, Arora M, Gennings C. Neonatal intensive care unit phthalate exposure and preterm infant neurobehavioral performance. PLoS ONE 2018; 13(3):e0193835.
- Verter F. Utah attempts to legalize placenta cell therapy. Parent's Guide to Cord Blood Foundation Newsletter Published 2024-04
- Verter F. Florida Stem Cell Law Flaunts the FDA. Parent's Guide to Cord Blood Foundation Newsletter Published 2025-06


