Jste zde
Lyophilization as an alternative to Cryopreservation
 Cell therapy is an application of viable cells with the intention of improving or curing medical conditions or diseases.1 Cell therapy is a key part of regenerative medicine that provides solutions to many conditions that otherwise cannot be addressed by medical devices, pharmaceuticals, and biological drugs. For example, hematopoietic stem cell transplantation (HSCT), using bone marrow or cord blood as a source of HSCs, is an established curative treatment for a broad variety of genetic, blood, and immune disorders.2
Cell therapy is an application of viable cells with the intention of improving or curing medical conditions or diseases.1 Cell therapy is a key part of regenerative medicine that provides solutions to many conditions that otherwise cannot be addressed by medical devices, pharmaceuticals, and biological drugs. For example, hematopoietic stem cell transplantation (HSCT), using bone marrow or cord blood as a source of HSCs, is an established curative treatment for a broad variety of genetic, blood, and immune disorders.2
Despite the high potential and over 200 years of experience with cell therapies, the number of available cellular treatments and their beneficial impact on patients are limited. Many applications are still emerging and remain at experimental stages. The challenges of developing a robust manufacturing process and the complex logistics for storage and distribution of cellular therapies play a significant role in delaying cell therapy progress.
Viable cells have short shelf-life at ambient temperatures that complicates their storage and delivery to patients. To overcome this shortcoming of cell therapies, scientists have developed cell and tissue preservation techniques to prolong life of living cells outside of the body.
In 1949, Christopher Polge, a graduate student at the National Institute for Medical Research in London, discovered a cryoprotective property of glycerol. He observed that when glycerol was added to a suspension of rooster spermatozoa before freezing to -80°C, most of the sperm cells retained normal motility upon thawing.3 Over the years since, more cryoprotective agents (CPAs) have been discovered.
Cryopreservation is a process that preserves cells and tissues by cooling the samples to very low temperatures. It is currently the only method allowing long-term storage of living cells and tissues for years to decades. The biology of cryopreserving cells is complex. Direct freezing damages the cell organelles and membrane due to ice crystal formation leading to cell death. The key to successful cryopreservation is to avoid damaging ice formation inside and outside the cells, which can be achieved by the use of CPAs.4
Currently, most of the samples stored in cell and tissue banks around the world are cryopreserved. Although cryopreservation retains viability of frozen cells, it requires specialized equipment to maintain ultralow temperatures. This limits widespread use of cell therapies, complicates distribution, and increases cost. Therefore, the development of preservation methods that allow long-term storage of viable cells at ambient temperatures has become an important goal for scientists working in the field of cellular therapies.
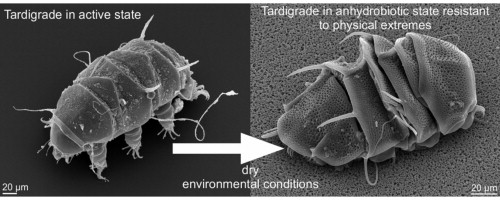 The existence of desiccation-tolerant anhydrobiotic animals like tardigrades suggests that dehydration and subsequent rehydration of mammalian cells should be possible.5 Tardigrades, or “water bears”, are near-microscopic animals with long, plump bodies, scrunched-up heads and eight legs. These tiny animals are almost indestructible and are even capable of surviving in outer space. They curl into a dehydrated ball by retracting their head and legs, protecting themselves within a glassy sugar goat. When reintroduced to water, the tardigrade can rehydrate back to life in just a few hours even after years of stasis.6
The existence of desiccation-tolerant anhydrobiotic animals like tardigrades suggests that dehydration and subsequent rehydration of mammalian cells should be possible.5 Tardigrades, or “water bears”, are near-microscopic animals with long, plump bodies, scrunched-up heads and eight legs. These tiny animals are almost indestructible and are even capable of surviving in outer space. They curl into a dehydrated ball by retracting their head and legs, protecting themselves within a glassy sugar goat. When reintroduced to water, the tardigrade can rehydrate back to life in just a few hours even after years of stasis.6
Understanding how organisms tolerate environmental extremes can provide strategies for preservation of mammalian cells and tissues. Several scientific reports in the literature have demonstrated feasibility of cell dehydration, however, data thus far has shown low cell viability and poor method reproducibility.6,7
Lyophilization (or freeze-drying) is the process of drying a frozen sample, from which frozen water is removed via sublimation, by transiting ice directly to the gas phase without passing through the intermediate liquid phase.8,9 Lyophilization has a long history of successful use in different areas of medicine and the food industry, but until recently lyophilization methods were not suitable for cell preservation.
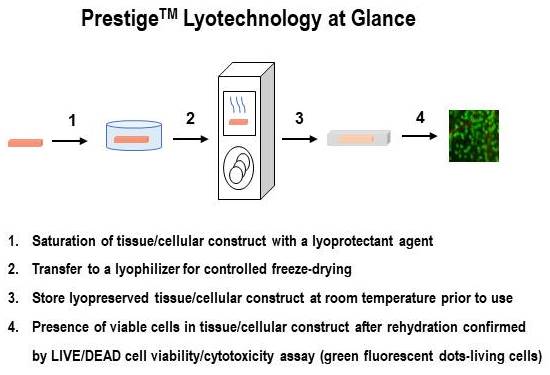
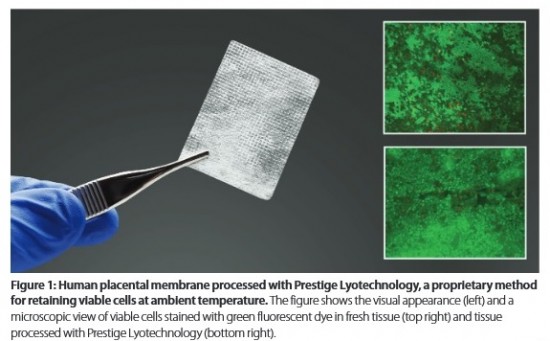 Osiris Therapeutics, Inc. has developed a lyophilization method that preserves living cells in tissues, allowing storage at room temperature for prolonged times without loss of cell viability. Preliminary results show that this method is reproducible, scalable, and can be applied to a variety of living cell and tissue types. The first commercial implementation of this lyotechnology will be for products containing placental cells.
Osiris Therapeutics, Inc. has developed a lyophilization method that preserves living cells in tissues, allowing storage at room temperature for prolonged times without loss of cell viability. Preliminary results show that this method is reproducible, scalable, and can be applied to a variety of living cell and tissue types. The first commercial implementation of this lyotechnology will be for products containing placental cells.
The full potential of lyotechnology is still under study and development. The ability of this method to preserve vascularized tissues and organs must be investigated. The optimization of cell manufacturing and cell storage with lyotechnology will take time. However, it is expected that lyotechnology will have a positive impact to expand the widespread use of cellular therapies, and may revolutionize cell and tissue banking.
References
- Mount NM, Ward SJ, Kefalas P, Hyllner J. Cell-based therapy technology classifications and translational challenges. Philosophical Transactions of the Royal Society B: Biological Sciences. 2015; 370(1680):20150017. doi:10.1098/rstb.2015.0017.
- Weissman IL, Shizuru JA. The origins of the identification and isolation of hematopoietic stem cells, and their capability to induce donor-specific transplantation tolerance and treat autoimmune diseases. Blood 2008; 112(9):3543–3553. doi:10.1182/blood-2008-08-078220
- Polge C, Smith AU, & Parkes, AS. Revival of Spermatozoa after Vitrification and Dehydration at Low Temperatures. Nature,1949; 164(4172), 666-666. doi:10.1038/164666a0
- Pegg, DE. (2014). Principles of Cryopreservation. Cryopreservation and Freeze-Drying Protocols Methods in Molecular Biology, Methods in Molecular Biology 2014; 1257:3-19
- Boothby, T. C., Piszkiewicz, S., Mehta, A., Brozena, A., Tapia, H., Koshland, D., . . . Pielak, G. (2017). Tardigrade Disordered Proteins Mediate Desiccation Tolerance. Biophysical Journal, 2017; 112(3) Supplement 1, p480a. http://dx.doi.org/10.1016/j.bpj.2016.11.2600
- Natan, D, Nagler, A, & Arav, A. Freeze-Drying of Mononuclear Cells Derived from Umbilical Cord Blood Followed by Colony Formation. PLoS ONE, 2009; 4(4). doi:10.1371/journal.pone.0005240
- Oliver, AE. Dry state preservation of nucleated cells: Progress and challenges. Biopreservation and Biobanking 2012; 10(4):376-85. doi:10.1089/bio.2012.0020.
- Innovation on the shelf: solving the puzzle of live-cell preservation. (2017, May). Retrieved from http://www.osiris.com/wp-content/uploads/2017/04/Nature-MedTech-Dealmakers-April-2017.pdf
- Morgan, C, Herman, N, White, P, & Vesey, G. Preservation of micro-organisms by drying; A review. Journal of Microbiological Methods, 2006; 66(2):183-193. doi:10.1016/j.mimet.2006.02.017



