Jste zde
Umbilical Cord Blood Bank to Fight HIV and AIDS
2022 Update: Story behind the 2022 announcement that a female patient had been cured of HIV after a cord blood transplant.
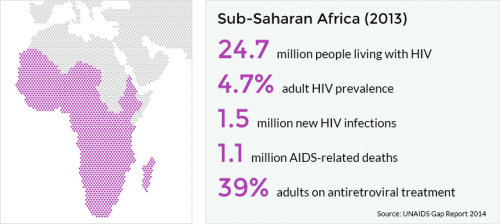 Worldwide, there are approximately 37 million people living with HIV, including more than 2 million children. South Africa is at the epicenter of this global scourge, with 1 in 5 people infected with HIV (AVERT, 2014).
Worldwide, there are approximately 37 million people living with HIV, including more than 2 million children. South Africa is at the epicenter of this global scourge, with 1 in 5 people infected with HIV (AVERT, 2014).
Many think that HIV/AIDS cannot be cured. It is true that there is no established and readily available cure of the disease. However, one man has had his infection suppressed to the point where there is no detectable level of virus in his blood. This is referred to as a functional cure. The man’s name is Timothy Ray Brown, also known as “the Berlin patient”.
Brown was an HIV-positive American man, living in Berlin, when he underwent a stem cell transplant in 2007 to treat leukaemia. He received stem cells from a donor who possessed a rare genetic mutation that provides immunity to HIV (Allers et al. 2011). The stem cells were transplanted successfully and repopulated his immune system. To this day, Timothy Ray Brown remains free of HIV even after he discontinued his antiretroviral treatment (Cohen 2011).
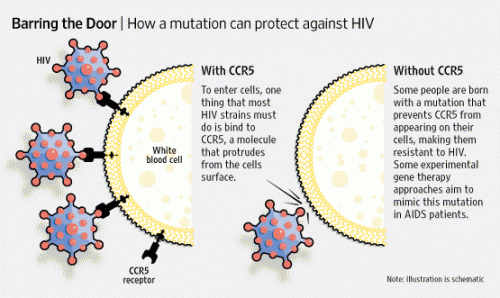 In order to understand how these stem cells were able to provide Brown with immunity to HIV, it is important to understand how HIV enters human cells. HIV-1, the most common HIV subtype, infects immune system cells by binding to a protein called CCR5 on the surface of the cells. A rare subset of the human population possess a natural mutation which prevents CCR5 from functioning and allowing the virus to enter the cell.
In order to understand how these stem cells were able to provide Brown with immunity to HIV, it is important to understand how HIV enters human cells. HIV-1, the most common HIV subtype, infects immune system cells by binding to a protein called CCR5 on the surface of the cells. A rare subset of the human population possess a natural mutation which prevents CCR5 from functioning and allowing the virus to enter the cell.
The case of Timothy Ray Brown sparked a flurry of scientists and doctors to attempt to recreate this functional cure on a bigger scale. In order to source these rare HIV-Immune cells, scientists began screening the general population for the naturally occurring mutation to request donations from these HIV resistant individuals. Because of the rarity of the mutation, it simply has not been feasible to recruit enough donors to provide matching stem cell transplants to HIV patients, who are a large and genetically diverse patient population.
A more novel approach is to start with healthy donors and modify their CCR5 gene so that their stem cells can provide disease resistance and immunity from future infection. Using newly improved and rapidly developing gene editing technologies, it is possible to edit the genome of cells that normally express CCR5 so that they produce mutant versions of this gene that are functionally inactive. Studies have shown that the introduction of this modification is possible in stem cells from adult blood. This approach of gene editing the CCR5 mutation eliminates the need to search for rare donors who naturally have the CCR5 mutation.
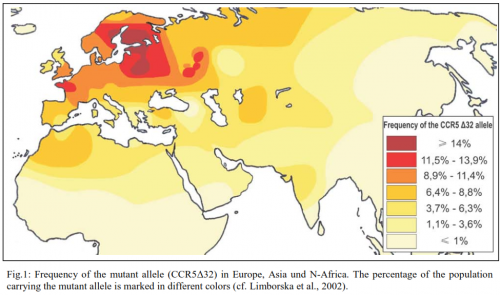 Tebas et al. 2014 proved that stem cell transplants in which the stem cells had been gene edited to disable CCR5 appear to be safe. Moreover they resulted in undetectable levels of HIV in one of four patients that were tested post-treatment.
Tebas et al. 2014 proved that stem cell transplants in which the stem cells had been gene edited to disable CCR5 appear to be safe. Moreover they resulted in undetectable levels of HIV in one of four patients that were tested post-treatment.
Although these results are encouraging, there are a number of challenges to using stem cell transplants as a cure for HIV among the general public. First is the availability of a matching donor for every HIV patient who needs stem cell therapy. A second, and more important challenge, is the stem cell transplant procedure itself. The chemotherapy required for this treatment is extremely dangerous and many life-threatening complications may arise. Given the improved efficacy of antiretroviral therapy in managing HIV disease, intense chemotherapy that obliterates the immune system is not justified when attempting to cure patients from their HIV infection (Cohen, 2011).
My hope is that improvements in transplant medicine will lead to safer stem cell transplants for HIV patients. If and when that occurs, a large scale bank of CCR5-negative stem cells will be required.
Singularity University, based in the heart of California’s Silicon Valley, holds a 10-week programme every year known as the Global Solutions Program (GSP). Applicants must propose a solution to one of humanity’s grand challenges, a solution that would positively influence the lives of a billion people in 10 years. If accepted into the programme, participants develop their ideas to the point where they can attract further investment from the biomedical research community and biotechnology industry.
 Singularity encourages applicants to “dream big” in the field of global health, which is of personal interest to me. Finding new cures is a passion of mine. Whilst working at Next Biosciences in South Africa, I developed the concept of establishing a bank of stem cells derived from umbilical cord blood that have been gene edited for resistance to HIV. Cord blood stem cells are readily available, easy to isolate, and are not ethically controversial. They are able to differentiate into immune cells and are safe for future transplantation. If cord blood stem cells are donated at birth and edited to exclude the expression of CCR5, they could provide a valuable resource in future HIV/AIDS therapy.
Singularity encourages applicants to “dream big” in the field of global health, which is of personal interest to me. Finding new cures is a passion of mine. Whilst working at Next Biosciences in South Africa, I developed the concept of establishing a bank of stem cells derived from umbilical cord blood that have been gene edited for resistance to HIV. Cord blood stem cells are readily available, easy to isolate, and are not ethically controversial. They are able to differentiate into immune cells and are safe for future transplantation. If cord blood stem cells are donated at birth and edited to exclude the expression of CCR5, they could provide a valuable resource in future HIV/AIDS therapy.
Earlier this year I entered the Rand Merchant Bank-sponsored Global Impact Challenge, a competition held in 17 countries around the world with first prize being a scholarship to attend Singularity University’s GSP16 to develop their idea. I was fortunate enough that my idea of a stem cell storage facility for gene edited umbilical cord blood-derived stem cells impressed the judges and I have been given the opportunity to attend this fantastic programme, beginning in June 2016.
References:
- Kristina Allers, Gero Hütter, Jörg Hofmann, Christoph Loddenkemper, Kathrin Rieger, Eckhard Thiel, and Thomas Schneider. Evidence for the cure of HIV infection by CCR5Δ32/Δ32 stem cell transplantation. 2010; 117(10):2791-2799 doi:10.1182/blood-2010-09-309591
- AVERT 2014 global HIV statistics. Accessed 12/05/2016 at: http://www.avert.org/professionals/hiv-around-world/global-statistics
- Jon Cohen. The Emerging Race to Cure HIV Infections. Science. Vol. 332, Issue 6031, pp. 784-789 2011. DOI:10.1126/science.332.6031.784
- Limborska S.A., Balanovsky O.P., Balanovskaya E.V., Slominsky P.A., Schadrina M.I., Livshits L.A., Kravchenko S.A., Pampuha V.M., Khusnutdinova E.K., Spitsyn V.A. Analysis of CCR5Δ32 Geographic Distribution and Its Correlation with Some Climatic and Geographic Factors. Hum Hered 2002; 53:49–54 DOI:10.1159/0000486
- Pablo Tebas, M.D., David Stein, M.D., Winson W. Tang, M.D., Ian Frank, M.D., Shelley Q. Wang, M.D., Gary Lee, Ph.D., S. Kaye Spratt, Ph.D., Richard T. Surosky, Ph.D., Martin A. Giedlin, Ph.D., Geoff Nichol, M.D., Michael C. Holmes, Ph.D., Philip D. Gregory, Ph.D., Dale G. Ando, M.D., Michael Kalos, Ph.D., Ronald G. Collman, M.D., Gwendolyn Binder-Scholl, Ph.D., Gabriela Plesa, M.D., Ph.D., Wei-Ting Hwang, Ph.D., Bruce L. Levine, Ph.D., and Carl H. June, M.D. Gene Editing of CCR5 in Autologous CD4 T Cells of Persons Infected with HIV. N Engl J Med 2014; 370:901-910 DOI:10.1056/NEJMoa1300662


