Jste zde
The use of umbilical cord MSC in China is booming
Most scientists think of mesenchymal stromal cells (MSC, or mesenchymal stem cells) as being associated with bone marrow or adipose tissue. However, new explorations of MSC derived from umbilical cord tissue (UC-MSC) promises abundance and great therapeutic value of these cells.
The obvious advantage of UC-MSC is the easy availability and abundance of inexpensive raw material (the umbilical cord), which could be utilized by cord blood banks and processing facilities for making of-the-shelf cellular products. Once you start to search for any type of data related to UC-MSC, you will be amazed how much has been done in China. I'd even say more - this is predominantly a "Chinese phenomenon".
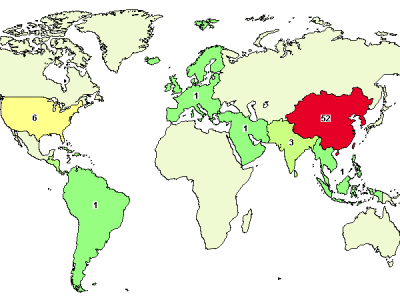
A search a query in ClinicalTrials.gov database for "umbilical cord mesenchymal stem" yields the above map of trial locations. If you read through the clinical trials and sort out some "noise", you will get about 70% (39 out of 56) of all worldwide UC-MSC trials attributed to China!
What is more exciting though, is that Chinese researchers are not only conducting the trials, but also publishing their results! In 2013 I was able to identify 8 clinical trials (not necessarily registered), whose results were published and indexed in the PubMed database. The clinical studies published by Chinese researchers assess UC-MSC for the conditions: autism, spinal cord injury, traumatic brain injury, type 1 diabetes, lupus, rheumatoid arthritis, aplastic anemia, primary biliary cirrhosis; as well as case reports for cerebral palsy and multiple sclerosis. The results of these studies are positive - meaning safety and feasibility were accomplished in Phase 1. Most of these reports finished with the phrase: "a larger, randomized controlled cohort study is warranted to confirm the clinical efficacy". However, such trials as rheumatoid arthritis (172 patients), autism (37 patients) and traumatic brain injury (40 patients) were designed to test efficacy and included control groups. According to their results, UC-MSC provided significant therapeutic benefit.
Yet another puzzle of the "Chinese phenomenon" is the lack of reporting in the Western mass media. None of those 8 published trials were picked up by media outlets and discussed publicly. This trend is opposite to the Western world, where any trial's report is highly discussed in mass media and among professionals. One of the possible (and probably the major) reasons for this is a common negative perception of the validity of Chinese science and clinical research among Westerners.
China is known to have a lack of enforcement of regulatory compliance; despite a government ban, clinics offering unproven stem cell "therapies" are rampant. Less stringent regulation of clinical trials in China could be a big reason for Western distrust of Chinese trial results. I'd argue, however, that professionals should read all published reports and rigorously analyze them, in order to judge the accuracy and validity of the "Chinese phenomenon". By discussing these Chinese results with peers, we can, at least, learn a lot from from their experience.